Glaucoma is a significant public health issue, and optometrists, as primary eye care clinicians, play an integral role in the diagnosis and management of the condition. As glaucoma is a chronic disease that has a nebulous definition, a successful glaucoma clinic requires the doctor to be proficient in a range of clinical skills and maintain continuing education in an evolving field; it also requires the doctor to have an efficient practice system in place. The successful clinic is rewarding, with many long-term patients, and the ability to prevent irreversible blindness. In this primer, we will be outlining some of the considerations for the early-career doctor who wants to develop their glaucoma clinic.
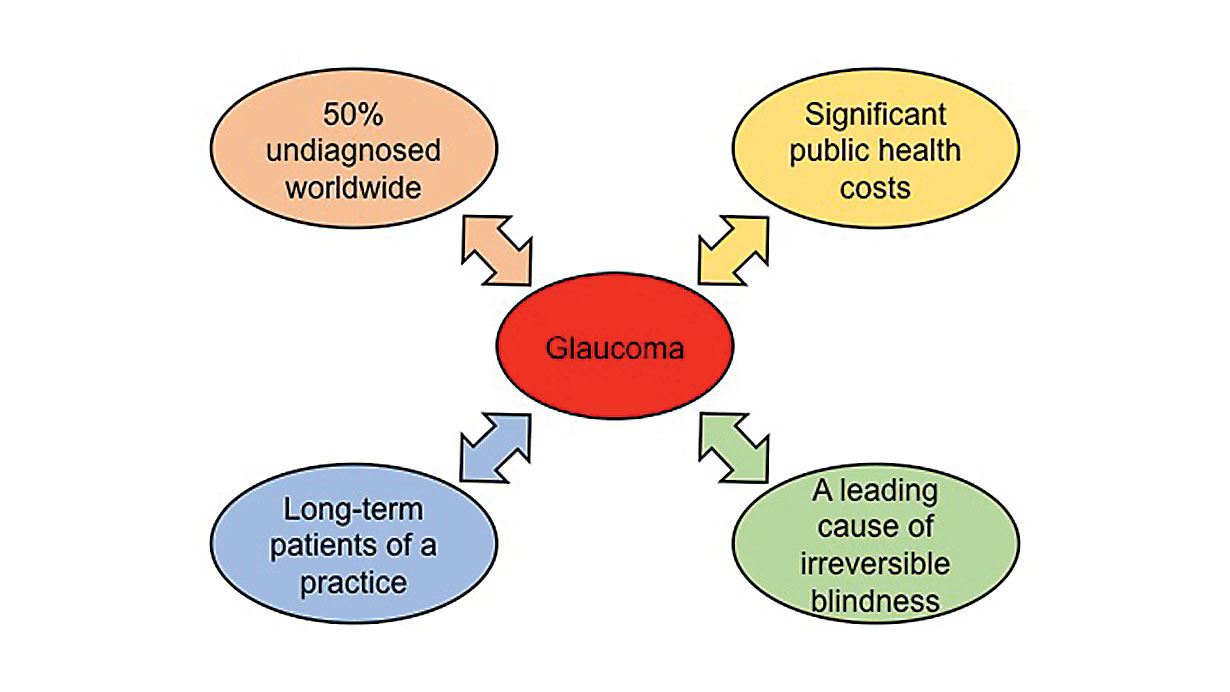 |
| Fig. 1. Glaucoma in the context of the current health care system. Click image to enlarge. |
Why Should We Care?
Glaucoma is one of the leading causes of irreversible blindness worldwide.1 With age being one of the primary risk factors for development of glaucoma, the aging and increasingly longevous populations in many countries creates a cumulative public health issue.1 As a chronic disease that requires close eye care, glaucoma can potentially occupy a lot of a clinician’s time and office space, and its treatment presents a significant cost to the health care system (Figure 1).2,3
Despite glaucoma’s prevalence and its dubious distinction as the most common optic neuropathy, diagnosing the disease is complex in some patients. The diagnosis of glaucoma requires careful examination of many clinical features: the optic nerve head, visual fields (VFs), intraocular pressure (IOP), anterior chamber angles and corneal thickness.4,5 Alongside these typical measurements, comprehensive history-taking can assess a patient’s risk profile. Cross-sectional data from a single examination, such as of the optic nerve head, IOP and VF, especially in the earliest stages of glaucoma, can provide only suspicious or borderline signs for glaucoma and can be inconclusive. Therefore, longitudinal information is often required, necessitating frequent reviews of these patients. As a result, it is unsurprising that over half of all cases of glaucoma are undiagnosed—and many are over- or misdiagnosed. This further confounds the problem posed by the disease.6-8
Unfortunately, despite advances in the field of glaucoma diagnostics, around half of all cases of initially diagnosed glaucoma are at the moderate stage or worse.9,10 This means, sadly, that many patients already have established, significant and often irreversible vision loss.
Despite these issues, glaucoma is a condition that, if managed correctly, can be professionally rewarding. In the sections below, we outline some of the essential tools and considerations for the eye doctor, especially those in the early stages of their career, to build their own glaucoma practice.
Clinical pearl: Glaucoma is a significant public health problem, but represents an opportunity for doctors to make an impact in patient care.
 |
| Click table to enlarge. |
Tools of the Trade
The advancement of imaging in this field has altered the optometrist’s approach to the diagnosis of glaucoma. In addition to taking history and symptoms, comprehensive glaucoma examination can be broken down into four key elements:
- IOP measurement and characterization of diurnal profile (including fluctuations)
- Assessment of visual function
- Examination of anterior eye structures
- Examination of posterior eye structures
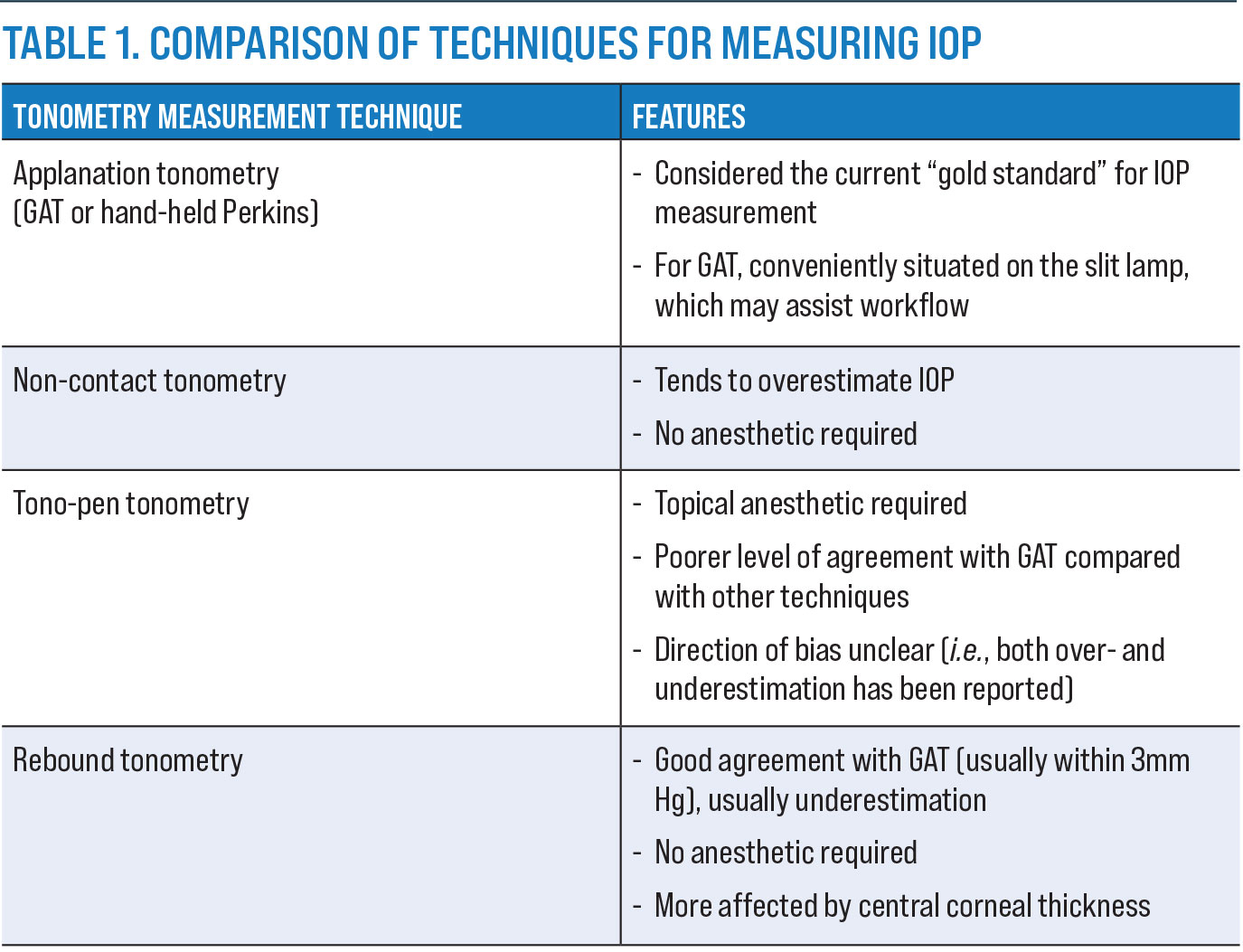
IOP measurement and profiling. The current standard for measurement of IOP in the management of glaucoma is Goldmann applanation tonometry (GAT).4,5 Other measurement techniques include non-contact tonometry, rebound tonometry and the Tono-pen (Table 1). Although these are also often deployed as mainstream clinical tools, they are often not directly comparable to Goldmann measurements, and their accuracy may also be affected by corneal parameters such as central corneal thickness. As such, these techniques should not be used interchangeably with GAT.11,12 Newer technologies such as methods accounting for corneal hysteresis may be available in some clinics, but this method’s role in the comanagement process is still evolving and the lack of agreement in corneal correction factors remain an issue.13,14
In recent years, there has been a shift away from single IOP measurements to instead characterization of the diurnal curve or fluctuations. Higher diurnal fluctuations are thought to be associated with an increased likelihood of disease progression.15,16 As many patients experience peak IOPs outside of typical office hours, 24-hour monitoring of IOP allows for detection of these peaks. Prior to the introduction of home-based monitoring devices such as the iCare Home, a patient-operated rebound tonometer, measurement of diurnal fluctuations would otherwise need to be performed in a setting requiring an overnight stay. These newer modalities allow for a more practical approach to implementation.17-19
In practices without access to patient-driven measurement devices, the water drinking test may be an alternative.20 This test measures the change in IOP following osmotic stress induced by the rapid ingestion of a defined quantity of water, usually 10mL per 1kg of body weight or a set amount of 800mL. Peak IOP measured during this test is thought to be representative of the eye’s aqueous outflow ability, whereby a higher peak pressure suggests a higher resistance to aqueous outflow.
Clinical pearl: Obtain IOP profiles for patients to assess baseline risk and treatment targets for future progression analysis.
Assessment of visual function. This step serves three key purposes in glaucoma care:
- Determining structure-function concordance. This relationship is a hallmark feature of glaucoma, and confirming it is important in the diagnostic step.21
- Disease staging and monitoring of progression. Most current glaucoma staging systems are based on VF parameters such as the mean deviation and the presence of central VF defects.22-24
- Assessing impact on quality of life. For example, central VF loss primarily affects near activities and social functioning while inferior peripheral VF loss affects distance activities and a patient’s sense of dependency more than superior peripheral VF loss.25,26
Aside from standard automated perimetry, alternative forms of perimetry are available. These include frequency-doubling technology, short-wavelength automated perimetry (“blue-on-yellow,” SWAP), flicker perimetry and edge perimetry.
The current recommendations from the American Academy of Ophthalmology state that white-on-white perimetry remains the preferred form of testing in glaucoma patients or suspects and do not advocate for the use of alternative perimetric methods.27,28 For initial examinations, the 24-2 is preferred for a comprehensive overview of common locations affected in the peripheral and central VF. The 10-2 is important for mapping central VF loss that may be missed by the 24-2.29,30 Clinicians need to use their judgment on which is most suitable for the individual patient.31,32
While some have advocated for obtaining at least six VF results in the first two years, in combining these pieces of evidence clinicians could consider performing more VF tests per visit to increase diagnostic confidence.33,34
More recently, a fast-test algorithm, SITA Faster, has become commercially available. This facilitates a paradigm shift towards more testing per visit, which would have been historically impractical with slower methods. However, clinicians need to remain vigilant of low test reliability results arising due to faster testing methods.35,36
Clinical pearl: White-on-white standard automated perimetry, performed preferably three or more times at each visit, is recommended for glaucoma assessment.
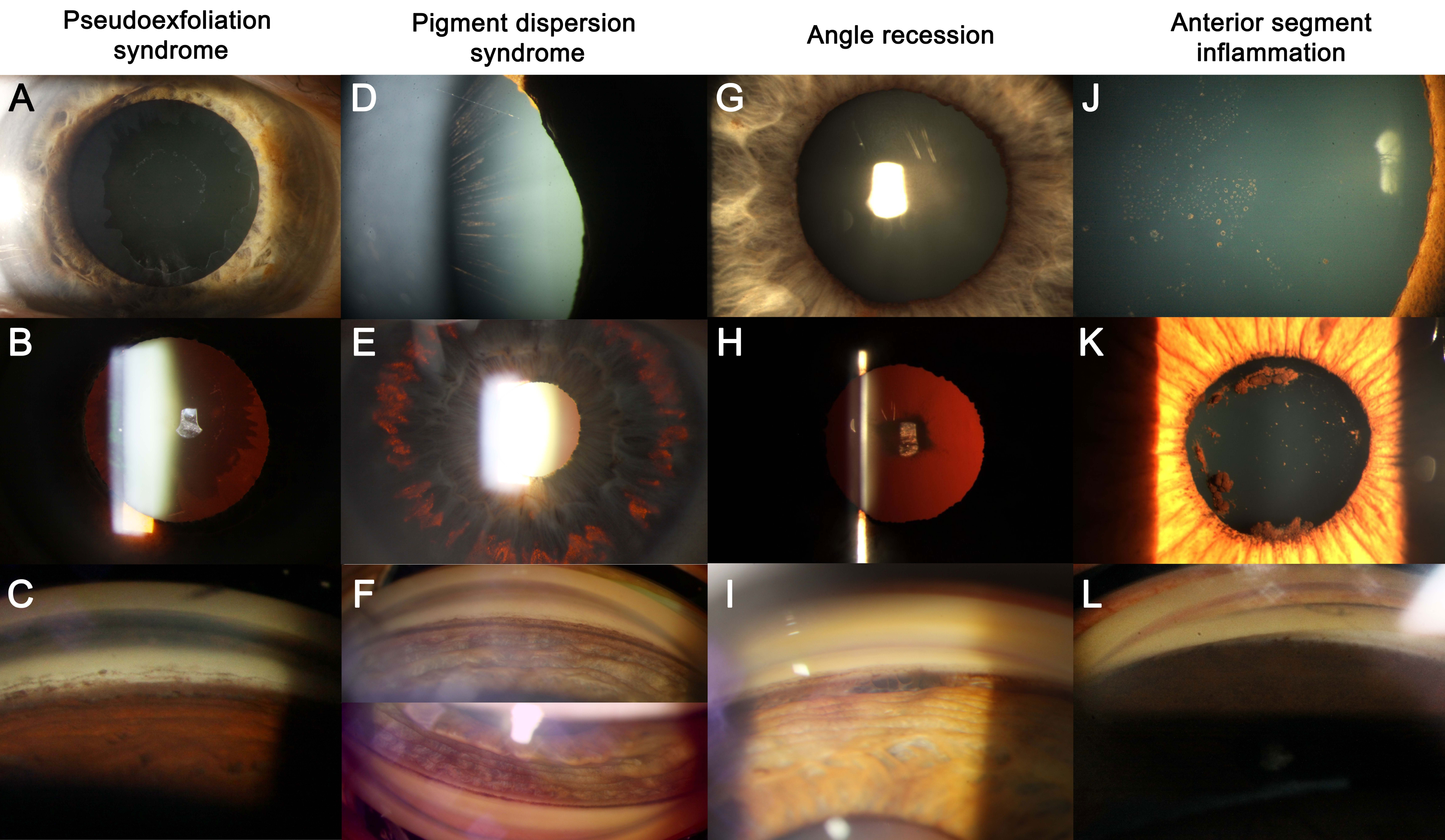 |
| Fig. 2. The slit lamp biomicroscopic and gonioscopic findings of psuedoexfoliation syndrome (A-C), pigment dispersion syndrome (D-F), angle recession (G-I) and anterior segment inflammation (J-L). (A) Extracellular material deposition in a double concentric bull’s eye pattern. (B) Transillumination view of the bull’s eye pattern. (C) Increased but patchy pigmentation of trabecular meshwork on gonioscopy. (D) Radial anterior lens surface pigment deposition. (E) Mid-peripheral iris transillumination defects. (F) Homogenous increased hyperpigmentation of the trabecular meshwork on gonioscopy. (G) Loss of the pupillary ruff. (H) Transillumination view of a traumatic cataract. (I) Angle recession on gonioscopy. (J) Pigmentation on the anterior surface of the lens capsule associated with a history of anterior uveitis. (K) Larger agglomerates of pigment on the anterior lens capsule surface suggestive of broken posterior synechiae. (L) Peripheral anterior synechiae with focal narrowing of the angle. Click image to enlarge. |
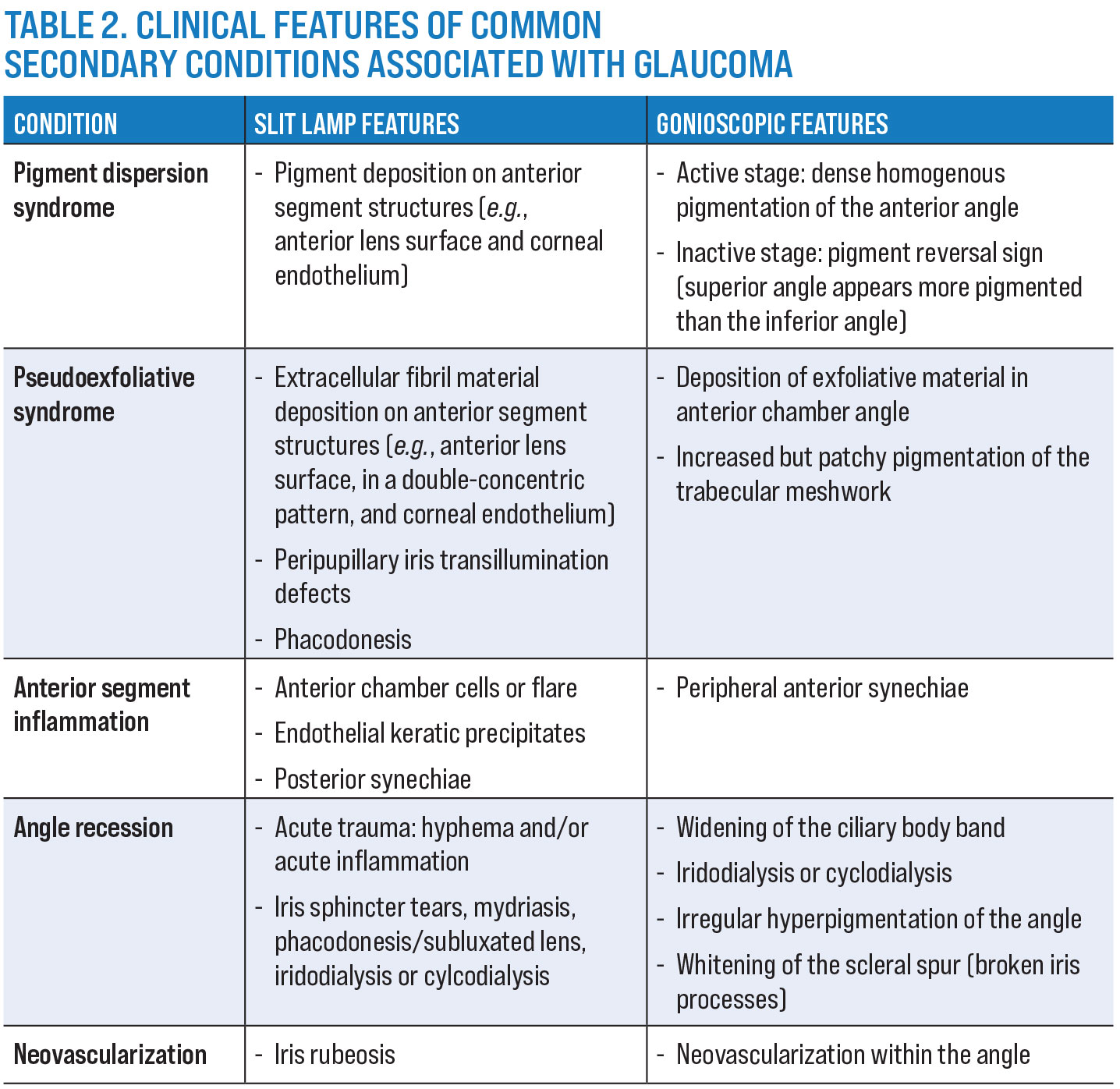
Examination of the anterior eye structures. Conditions affecting the anterior segment may contribute to the development of glaucoma. Secondary conditions such as pigment dispersion syndrome, pseudoexfoliative syndrome, anterior segment inflammation and angle recession can result in elevation of IOP and subsequently glaucomatous damage to the optic nerve head (Figure 2).
A summary of the slit lamp findings of common secondary conditions is shown in Table 2. These conditions also have the potential to influence treatment selection. For example, clinicians may elect for topical treatment rather than selective laser trabeculoplasty first-line therapy in cases of angle recession glaucoma, while a patient with primary open-angle glaucoma may be a more suitable candidate for laser treatment.
Corneal endothelial dystrophies may lead to clinicians being more cautious with carbonic anhydrase inhibitors. Patients with iris or skin configurations that may be more susceptible to cosmetic changes arising from prostaglandin therapy should be carefully warned. Thus, detailed assessment of the anterior segment using slit lamp biomicroscopy, both prior to and following pupil dilation, forms an essential component of the glaucoma assessment.
Clinical pearl: Assess the anterior segment for secondary causes of glaucoma.
Assessment of the anterior chamber angle. Gonioscopy remains the gold standard for anterior angle assessment.37 Unlike van Herick angle estimation or anterior segment optical coherence tomography (OCT), gonioscopy is not affected by ocular surface diseases such as pterygia or other limbal opacities.
To maximize the direct and dynamic visualization offered by gonioscopy, perform both primary gaze and off-axis evaluations. This allows for distinction between true narrow angles vs. open angles that appear narrow in primary gaze due to a steep iris configuration. In cases where the angle appears narrow despite off-axis viewing, perform indentation, which allows for differentiation between appositional closure (i.e., widens with indentation) and mechanical closure due to the presence of synechiae or neovascularization (i.e., no deepening despite indentation). It is also useful for differentiating between iris processes and synechiae. Gonioscopy also provides an in vivo view of the angle important for identifying secondary causes of glaucoma like pigment dispersion syndrome and angle recession.
Anterior segment OCT is an emerging technology for assessing the anterior chamber angle. While it offers a noninvasive method of visualizing the angle structures such as Schwable’s line, Schlemm’s canal, the scleral spur and the ciliary body, it is not able to distinguish between the pigmented and non-pigmented aspects of the trabecular meshwork. There are no widely accepted cut-offs with acceptable sensitivity and specificity to differentiate between disease and normal.38 Other anterior segment imaging modalities include Scheimpflug imaging and ultrasound biomicroscopy, which have their own specific advantages.39
Clinical pearl: Gonioscopy is critical for comprehensive assessment of the anterior chamber angle.
Examination of the posterior eye structures. The current clinical standard for visualizing characteristic optic nerve head features of glaucoma is using stereoscopic funduscopy. Adding a red-free filter during funduscopic assessment or through retinal camera software allows for improved assessment of the retinal nerve fiber layer (RNFL) integrity, highlighting regions of diffuse or focal structural loss. Pharmacologic mydriasis can enhance visualization of the optic nerve by improving assessment of the cup size and depth, which is best appreciated stereoscopically. Retinal photography allows for serial documentation of the disc and peripapillary appearance, useful for detecting change through flicker stereophotography of the nerve.40,41 Unlike some imaging modalities, fundus photography can be largely agnostic to the hardware and software and so is highly conducive to long-term monitoring of the disc appearance.
Clinical pearl: The current clinical standard is stereoscopic examination of the optic nerve head and adjacent retinal structures. Pharmacologic mydriasis is also recommended.
 |
| Click table to enlarge. |
Ancillary imaging in the optic nerve head and retinal examination. OCT has become a practice staple for the diagnosis and management of glaucoma.42 It offers a quantitative method for assessing glaucomatous changes such as changes to the physiological cup, neuroretinal rim loss or thinning of the neural layers. Of note, OCT is useful for early detection of glaucomatous progression that may not be appreciable based on funduscopic information alone. In addition to this, it allows for visualization of features previously thought to be “invisible” such as the anatomical disc margin (the termination of the scleral canal) and the lamina cribrosa.
As well as imaging of the optic nerve head and the adjacent structures, imaging of the macula in glaucoma is important to identify structural losses occurring in glaucoma. Significantly, approximately half of the ganglion cell bodies are located within the macula, and imaging in this region highlights relevant pathological changes across the spectrum of glaucoma stages. It is important to note for clinicians that structure-function correlations need to be made with the appropriate regions in visual space, i.e., that structural loss in the macula needs to be assessed in conjunction with a suitable central VF testing grid.
Despite these advantages, use of OCT in glaucoma is not without its limitations. When starting out, it is easy for clinicians to rely on the red and green coding from instrument printouts as a guide to differentiating between normal and abnormal findings.43 Clinicians must carefully interpret and scrutinize the data and place it in the context of the other clinical results. In some cases, the raw data (i.e., heat map, TSNIT curves) may be more useful than relying on normative comparisons alone.
Another limitation of OCT is the “measurement floor” encountered in advanced stages of disease whereby the extent of structural loss such as the RNFL thickness, exceeds the lowest measurable thickness and the remaining “thickness” is due to non-neuronal structures, such as glial tissue or the retinal vasculature.44 As such, OCT may have limited utility in later stages of disease.
Finally, the OCT features of glaucoma overlap significantly with those of other optic nerve conditions such as ischemic or compressive optic neuropathies. Subsequently, OCT alone cannot be used to diagnose or screen for glaucoma and must be used in conjunction with other tests.
Clinical pearl: Ancillary imaging of the optic nerve head, such as OCT, can reveal additional, important details for cross-sectional diagnosis and progression analysis. The tools of the trade in a glaucoma practice are rarely used in isolation and are best maximized when applied as using a complementary approach.
 |
| Click table to enlarge. |
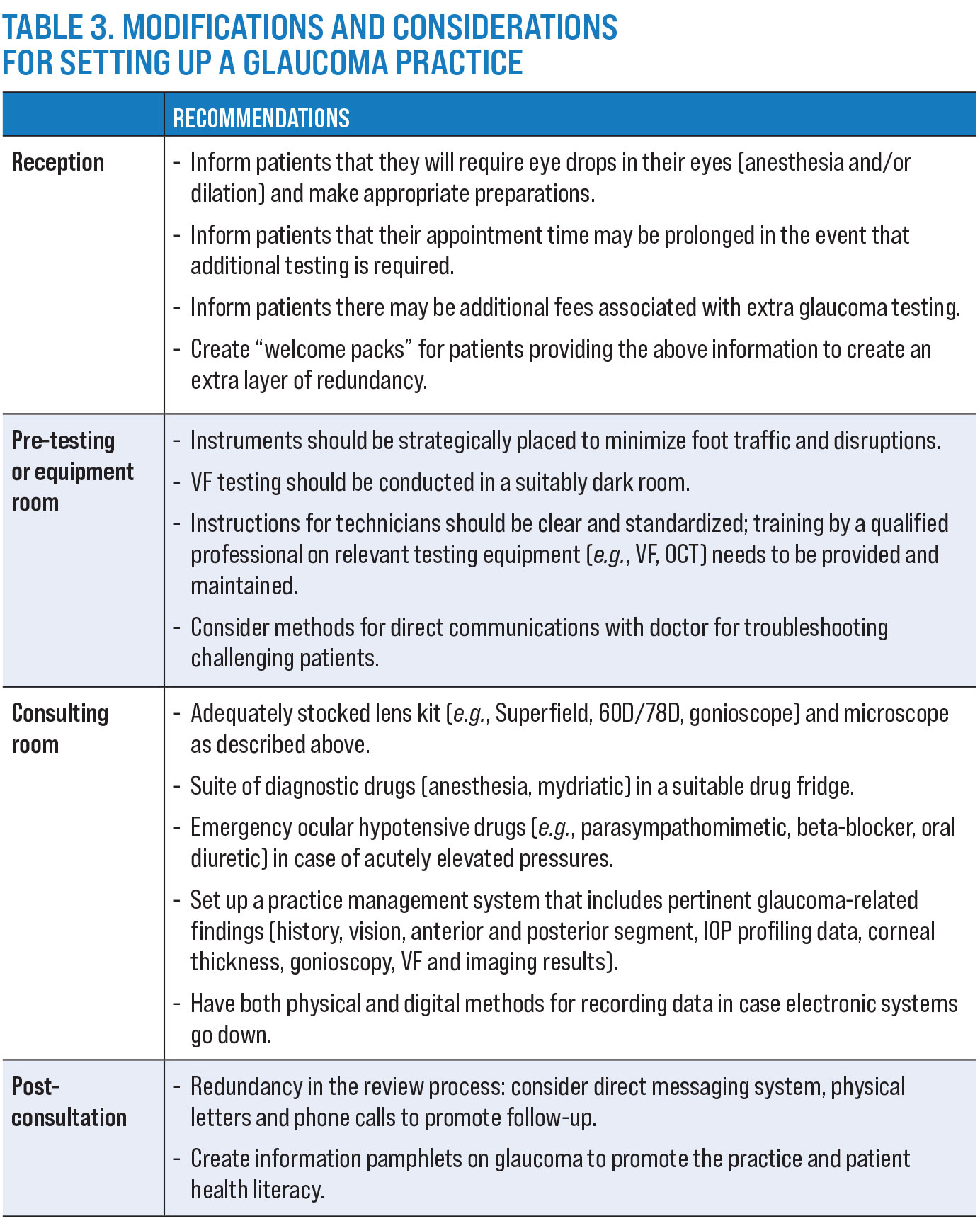
Set Up Your Glaucoma Practice
So, as a doctor interested in setting up a practice to manage glaucoma, what kinds of preparations do you need? This question can be examined from the front of the practice all the way to the back in the clinician’s office. Some of these considerations are shown in Table 3.
Doctors will find that while there can be big changes to the way a practice operates to meet the demands of glaucoma patients—such as a robust recall system, patient information handouts and separate testing rooms—many such modifications can be easily translatable to other conditions of the eye, aiding the practice as a whole. The workflow can be individualized to the doctor’s clinic but should ultimately complete the loop for excellent patient care.
It is important to create layers within the practice that will promote the practice and improve patient health literacy. Many patients will be unaware that you offer comprehensive glaucoma (or other specialized) services. Strategies to change this will help the doctor build their practice and present an impression of professionalism.
Takeaways
There are a lot of considerations when setting up a clinic to provide glaucoma care. However, given the breadth of the public health issue, it is very rewarding to be able to serve a population of patients who require ongoing eye care.
Now that we have set up a glaucoma clinic, the next step is to consider processes for providing glaucoma care in the consulting room. In part two of this series, to be published next month, we will outline the process of glaucoma care by the OD, including diagnosis, identification of progression and management.
Dr. Phu is a clinician-scientist with academic and clinical positions at the Centre for Eye Health in Kensington, New South Wales, Australia, and the School of Optometry and Vision Science, University of New South Wales. He is a Fellow of the American Academy of Optometry and a Diplomate in glaucoma. Dr. Wang is a research and clinical staff optometrist at the Centre for Eye Health. She is a Fellow of the AAO. They have no financial interests to disclose.
| 1. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081-90. 2. Varma R, Lee PP, Goldberg I, et al. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515-22. 3. Dirani M, Crowston JG, Taylor PS, et al. Economic impact of primary open-angle glaucoma in Australia. Clin Exp Ophthalmol. 2011;39:623-32. 4. Gedde SJ, Lind JT, Wright MM, et al. Primary open-angle glaucoma suspect Preferred Practice Pattern. Ophthalmology. 2021;128:P151-92. 5. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma Preferred Practice Pattern. Ophthalmology. 2021;128:P71-150. 6. Chua J, Baskaran M, Ong PG, et al. Prevalence, risk factors, and visual features of undiagnosed glaucoma: the Singapore Epidemiology of Eye Diseases Study. JAMA Ophthalmol. 2015;133:938-46. 7. Mitchell P, Smith W, Attebo K, et al. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996;103:1661-1669. 8. Heijl A, Bengtsson B, Oskarsdottir SE. Prevalence and severity of undetected manifest glaucoma: results from the early manifest glaucoma trial screening. Ophthalmology. 2013;120:1541-5. 9. Boodhna T, Saunders LJ, Crabb DP. Are rates of vision loss in patients in English glaucoma clinics slowing down over time? trends from a decade of data. Eye (Lond). 2015;29:1639. 10. Boodhna T, Crabb DP. Disease severity in newly diagnosed glaucoma patients with visual field loss: trends from more than a decade of data. Ophthalmic Physiol Opt. 2015;35:225-30. 11. Rodter TH, Knippschild S, Baulig C, et al. Meta-analysis of the concordance of Icare Pro-based rebound and Goldmann applanation tonometry in glaucoma patients. Eur J Ophthalmol. 2020;30:245-52. 12. Demirci G, Erdur SK, Tanriverdi C, et al. Comparison of rebound tonometry and non-contact airpuff tonometry to Goldmann applanation tonometry. Ther Adv Ophthalmol. 2019;11:2515841419835731. 13. Wachtl J, Toteberg-Harms M, Frimmel S, et al. Correlation between dynamic contour tonometry, uncorrected and corrected Goldmann applanation tonometry and stage of glaucoma. JAMA Ophthalmol. 2017;135:601-8. 14. Katsimpris JM, Theoulakis PE, Vasilopoulos K, et al. Correlation between central corneal thickness and intraocular pressure measured by Goldmann applanation tonometry or pascal dynamic contour tonometry. Klin Monbl Augenheilkd. 2015;232:414-8. 15. Matlach J, Bender S, Konig J, et al. Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression. Clin Ophthalmol. 2019;13:9-16. 16. Kim SH, Lee EJ, Han JC, et al. The effect of diurnal fluctuation in intraocular pressure on the evaluation of risk factors of progression in normal tension glaucoma. PLoS One. 2016;11:e0164876. 17. Huang J, Katalinic P, Kalloniatis M et al. Diurnal intraocular pressure fluctuations with self-tonometry in glaucoma patients and suspects: a clinical trial. Optom Vis Sci. 2018;95:88-95. 18. Rojas CD, Reed DM, Moroi SE. Usefulness of Icare Home in telemedicine workflow to detect real-world intraocular pressure response to glaucoma medication change. Ophthalmol Glaucoma. 2020;3:403-5. 19. Sood V, Ramanathan US. Self-monitoring of intraocular pressure outside of normal office hours using rebound tonometry: initial clinical experience in patients with normal tension glaucoma. J Glaucoma. 2016;25:807-11. 20. Susanna R, Jr., Clement C, Goldberg I, et al. Applications of the water drinking test in glaucoma management. Clin Exp Ophthalmol. 2017;45:625-31. 21. Iyer J, Vianna JR, Chauhan BC, et al. Toward a new definition of glaucomatous optic neuropathy for clinical research. Curr Opin Ophthalmol. 2020;31:85-90. 22. De Moraes CG, Sun A, Jarukasetphon R, et al. Association of macular visual field measurements with glaucoma staging systems. JAMA Ophthalmol. 2019;137:139-45. 23. Sankar PS, O’Keefe L, Choi D, et al. The SCHEIE visual field grading system. J Clin Exp Ophthalmol. 2017;8. 24. Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141:24-30. 25. Chun YS, Sung KR, Park CK, et al. Vision-related quality of life according to location of visual field loss in patients with glaucoma. Acta Ophthalmol 2019;97:e772-9. 26. Kulkarni KM, Mayer JR, Lorenzana LL, et al. Visual field staging systems in glaucoma and the activities of daily living. Am J Ophthalmol. 2012;154:445-51,e443. 27. Delgado MF, Nguyen NT, Cox TA, et al. Automated perimetry: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:2362-74. 28. Jampel HD, Singh K, Lin SC, et al. Assessment of visual function in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:986-1002. 29. De Moraes CG, Hood DC, Thenappan A, et al. 24-2 visual fields miss central defects shown on 10-2 tests in glaucoma suspects, ocular hypertensives and early glaucoma. Ophthalmology 2017;124:1449-56. 30. Sullivan-Mee M, Karin Tran MT, Pensyl D, et al. Prevalence, features and severity of glaucomatous visual field loss measured with the 10-2 achromatic threshold visual field test. Am J Ophthalmol. 2016;168:40-51. 31. West ME, Sharpe GP, Hutchison DM, et al. Value of 10-2 visual field testing in glaucoma patients with early 24-2 visual field loss. Ophthalmology. September 6, 2020.[Epub ahead of print]. 32. Wu Z, Medeiros FA, Weinreb, RN et al. Performance of the 10-2 and 24-2 visual field tests for detecting central visual field abnormalities in glaucoma. Am J Ophthalmol. 2018;196:10-7. 33. Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569-73. 34. Crabb DP, Garway-Heath DF. Intervals between visual field tests when monitoring the glaucomatous patient: wait-and-see approach. Invest Ophthalmol Vis Sci. 2012;53:2770-6. 35. Heijl A, Patella VM, Chong LX, et al. A new SITA perimetric threshold testing algorithm: construction and a multicenter clinical study. Am J Ophthalmol. 2019;198:154-65. 36. Phu J, Khuu SK, Agar A, Kalloniatis M. Clinical Evaluation of Swedish Interactive Thresholding Algorithm-Faster compared with Swedish Interactive Thresholding Algorithm-Standard in normal subjects, glaucoma suspects and patients with glaucoma. Am J Ophthalmol. 2019;208:251-64. 37. Smith SD, Singh K, Lin SC, et al. Evaluation of the anterior chamber angle in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120:1985-97. 38. Phu J, Wong B, Lim T, et al. Assessment of angle closure spectrum disease as a continuum of change using gonioscopy and anterior segment optical coherence tomography. Ophthalmic Physiol Opt. 2020;40:617-31. 39. Riva I, Micheletti E, Oddone F, et al. Anterior chamber angle assessment techniques: a review. J Clin Med. 2020;9. 40. Ikram MK, Borger PH, Assink JJ, et al. Comparing ophthalmoscopy, slide viewing, and semiautomated systems in optic disc morphometry. Ophthalmology. 2002;109:486-93. 41. Ahn J, Yun IS, Yoo HG, et al. Developing new automated alternation flicker using optic disc photography for the detection of glaucoma progression. Eye (Lond) 2017;31:119-26. 42. Ly A, Phu J, Katalinic P, et al. An evidence-based approach to the routine use of optical coherence tomography. Clin Exp Optom. 2019;102:242-59. 43. Chong GT, Lee RK. Glaucoma vs. red disease: imaging and glaucoma diagnosis. Curr Opin Ophthalmol 2012;23:79-88. 44. Moghimi S, Bowd C, Zangwill LM, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019;126:980-8. |

