
The campaign against invading microbial agents is common to all health-care practitioners. Of concern to optometrists: the invasion of the cornea by various microorganisms, which results in microbial keratitis (MK).
Management of MK is particularly challenging due to increased resistance to second- and third-generation fluoroquinolones and the emergence of atypical microbes. Also, management strategies for some types of MK are controversial. Here, well examine various treatment options.
What is MK?
MK is characterized by excavation of the corneal epithelium, Bowmans layer and stroma with infiltration and necrosis of tissue.1 The most common presenting symptom of MK is acute onset of a red, painful eye, with or without discharge.
Although contact lens wear is a well-known risk factor for MK, ocular trauma is another common cause. Other risk factors include ocular surgery, use of topical corticosteroids, ocular surface disease, lid misalignment, smoking and systemic diseases such as diabetes mellitus.2
Nearly one-third of MK lesions are located peripherally, and another third are mid-peripheral. Fewer than one in five lesions are centrally located, yet these have the poorest prognosis because the resulting scar affects the line of sight. Even fewer lesions are found in multiple quadrants.
MK damages the eye in two ways: the direct physical impact of the replicating microorganism and the infected individuals immune response, which is activated when the infection occurs.
Inflammatory cytokines, chemokines, polymorphonuclear leukocytes and T cells work to destroy the invading microbes, but they cause liquefactive necrosis of the surrounding tissue, resulting in further structural damage to the cornea.3 Left untreated, the patients eye can be permanently impaired because the patients immune response may result in corneal scarring and permanent vision loss.
Antimicrobials are only designed to neutralize the replicating microorganisms; they have no direct effect on the patients inflammatory response. So, topical corticosteroids are often required as supplementary treatment to limit the consequences of inflammation. The therapeutic challenge lies in creating a balance between the management of the initial infection and the subsequent inflammation; this delicate balance varies from microbe to microbe and from patient to patient.

When patients present with suspected microbial keratitis, you must question them about possible trauma (especially with vegetative material), contact lens wear, and recent travel. Your examination should include a careful evaluation of the bulbar and palpebral conjunctiva for injection or nodules, the anterior chamber for inflammation, and the cornea for defects and staining patterns. Dont overlook measurement of the size and depth of any corneal defect; some microbial infections display characteristic findings that help determine a specific etiology (see Characteristic Findings in Common Types of Microbial Keratitis).
The longstanding practice has been to obtain cultures in any case of suspected MK that involves the visual axis; demonstrates significant depth within the stroma; is associated with significant inflammation, hypopyon and/or necrosis; or if the patients history is suspicious for MK.4
But, during the 1990s, newer antibiotics were introduced with enhanced antimicrobial effectiveness, so culturing of MK was done less frequently. With increased bacterial resistance to medications, however, and with the resurgence of some unusual infectious agents (such as Fusarium), we should again consider culturing nosocomial infections in immunocompromised individuals, traumatic ulcers that involve vegetative matter, and all lesions that involve the line of sight and are severe or non-responsive to therapy. These conditions have a poorer prognosis and carry a greater risk of complications.
Be advised: Conventional wisdom has held that cultures are not conclusive once therapy is initiated. In one study of 334 patients, cultures were positive in 44% of individuals who had begun topical therapy vs. 38% of patients who had not begun topical therapy.5 It generally takes a longer time to culture organisms if a patient was previously treated topically.
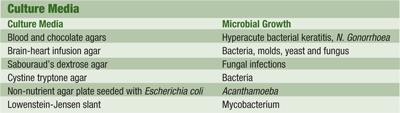
The organisms drug sensitivity may also be measured by the Kirby-Bauer disc diffusion method. Because this method determines levels based on serum instead of at the cornea, it tends to overestimate resistance for topical therapy.6 The culturing medium is important in the timely recognition of infectious agents and may help differentiate among the etiologies of microbial keratitis. Different organisms require different media to plate out (see Culture Media).
Typical findings associated with bacterial keratitis include pain, generalized redness, and the presence of an epithelial defect with stromal progression, often with an overlying mucopurulent response. Additionally, anterior chamber reactions are relatively common with bacterial keratitis.
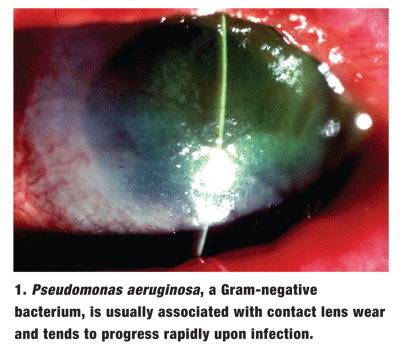
In one retrospective study, Pseudomonas aeruginosa was the most common cultured Gram-negative organism, followed by Serratia marcescens, with equal distribution among contact lens patients.7 Serratia marcescens has an affinity for irregular corneal surface conditions, such as those that result from penetrating keratoplasty.
Pseudomonas aeruginosa (figure 1) is of particular concern because of its accelerated corneal penetration capabilities. This opportunistic organism is strongly associated with contact lens wear and usually progresses rapidly after the initial insult.
Numerous other bacterial organisms are also associated with MK. The treatment protocol for all cases of MK involves removing and/or neutralizing the microbe and reducing the inflammatory response.
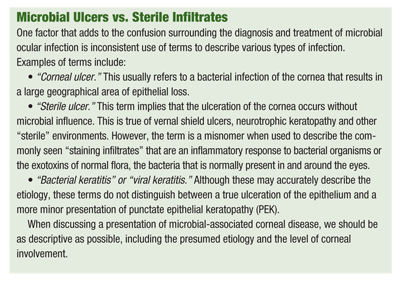
Once you determine that a keratitis is caused by true bacterial infiltration vs. the more common secondary sterile inflammatory response, various antibiotics are available. Many aspects of the patients status should be considered when determining which treatment option is most appropriate; these include the depth, size and location of the lesion within the cornea (see Microbial Ulcers vs. Sterile Infiltrates).
Before fluoroquinolones became commercially available, fortified topical aminoglycosides (such as tobramycin) were the mainstay of treatment for bacterial keratitis. In cases of severe bacterial infection, some O.D.s still prefer these agents. The aminoglycosides are bactericidal, inhibiting protein synthesis. They have especially good coverage against Gram-negative bacteria, including the Pseudomonas species, as well as several Gram-positive species.
Topical formulations of tobramycin and gentamicin are available in solution and ointment for bacterial infections of the lids and conjunctiva and as prophylactic treatment for the cornea. When treating significant bacterial keratitis, such as an ulcer, fortified formulations are recommended. These formulas can be difficult to obtain, however, and side effects of these preparations include corneal and surrounding tissue toxicity, injection of the inferior cul-de-sac, and edema of the lids. Other medications that may be compounded by a pharmacist and used in the treatment of bacterial keratitis include vancomycin and the cephalosporins.
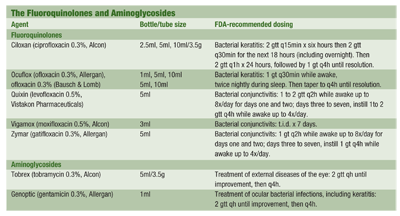
Fluoroquinolones are often the first choice for treating bacterial keratitis due to their rapid bactericidal characteristics and easy availability. Five topical fluoroquinolones are available, although only Ciloxan (ciprofloxacin, Alcon) and Ocuflox (ofloxacin 0.3%, Allergan) are FDA-approved for treating true bacterial ulcers. However, bacterial resistance to these medications has increased from widespread use, and the bacteria that are generally resistant to ciprofloxacin and ofloxacin may also be resistant to levofloxacin.8-11
Further, ciprofloxacin has been shown to result in crystalline deposits within the ulcer bed without negative sequelae. Finally, all the fluoroquinolones demonstrate mild corneal toxicity over time but to a less significant degree than the aminoglycosides.
Although the fourth-generation fluoroquinolones, namely Vigamox (moxifloxacin, Alcon) and Zymar (gatifloxacin, Allergan), are only FDA-approved for treating bacterial conjunctivitis, they also have changed the way we treat corneal ulcers. These drugs provide enhanced coverage of Gram-positive bacteria and anaerobic and atypical pathogens, and there is less likelihood of bacterial resistance.
Vigamox is self-preserved and has a limited bottle size (3ml). Zymar is BAK-preserved and available in a larger bottle size (5ml). Vigamox has slightly better penetration and has demonstrated antimicrobial activity beyond bacteria alone.12
Whichever antibiotic you choose, the dosing schedule is especially important. FDA guidelines are specific for the treatment of true bacterial ulcers using ciprofloxacin and ofloxacin (see The Fluoroquinolones and Aminoglycosides), but specific dosing protocols for treating ulcers using the fourth-generation fluoroquinolones have not been established. So, if you choose fourth-generation fluoroquinolones for treatment of a true bacterial corneal ulcer, you must obtain the patients informed consent, since it would be an off-label use.
Determine the schedule for your patient according to the severity of corneal compromise. In-office loading doses of fourth-generation fluoroquinolones are recommended initially for treatment of severe bacterial keratitis, including true ulcers, with administration of an appropriate antibiotic every five to 10 minutes for 30 to 60 minutes. More moderate infections may require a dosing frequency of every two hours.
As the infection resolves, you can modify the schedule, decreasing dosing frequency to limit side effects but not to a point that might foster resistance. In general, dosing should not drop below q.i.d. to reduce the likelihood of the development of bacterial resistance. Dosing should continue throughout a course of corticosteroids if initiated.
Concurrent oral doxycycline may be helpful for patients with true bacterial keratitis since it acts against collagenase for an anti-inflammatory effect.13 A treatment regimen of 100mg doxycycline q.d. for one to two weeks may reduce the risk of corneal melting.
Topical corticosteroids also play an important role in the management of these cases, as we will discuss later.
Viral Infections
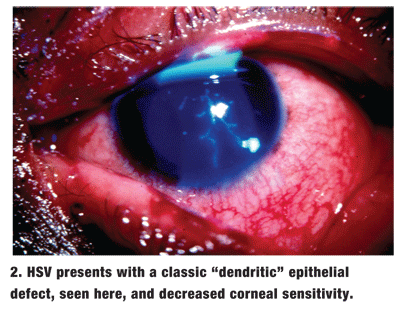
Among the viral organisms that can cause MK, herpes simplex virus (HSV) poses the highest threat to the cornea. Individuals who have HSV present with an acute, unilateral red eye accompanied by serous discharge, preauricular lymphadenopathy, decreased corneal sensitivity and a classic dendritic epithelial defect (figure 2). Dendrites in HSV may not appear until a few days into the infection, so patients may initially present with non-specific punctate epithelial keratitis (PEK). HSV dendritiform lesions are truly of an ulcerative nature, which equates to tissue necrosis/loss in the area of the lesion. Be careful: There are mimickers that present as pseudo-dendrites, but these lesions tend to be flat or elevatednot depressed, as an ulcer would be.
To differentiate HSV epithelial keratitis from other branching fluorescein staining patterns, look for true end-bulbs that stain positively with rose bengal. HSV is a recurrent disease that has a cautious long-term visual prognosis; compromise to the corneal structure is likely.
Although adenovirus, varicella zoster and other viral organisms can affect the cornea, they generally do not pose a significant threat to its integrity and clarity. In contrast, HSV is a frequent cause of corneal opacification.
HSV epithelial keratitis is treated according to a well-established protocol. Viroptic (trifluridine 1%, Monarch) is prescribed q2h while awake, up to nine times per day at initial presentation. Due to the toxic nature of trifluridine, taper the dosing frequency according to the patients response, often dropping quickly to q.i.d. Continue this schedule for three to five days following complete re-epithelialization.
Besides topical medications for the treatment of HSV keratitis, in-office epithelial debridement may significantly reduce the viral load on the cornea. Remember: When debriding the cornea, move from good tissue toward bad tissue to avoid unnecessarily enlarging the defect. Immediately apply trifluridine following debridement, then have the patient begin a regular dosing pattern.
Zovirax (acyclovir, GlaxoSmithKline) is the traditional oral medication used for treatment of herpetic infections. A dosage of 400mg five times a day for seven to 10 days is recommended for HSV keratitis and is most beneficial when started early in the disease process.14 Side effects of acyclovir include nausea, vomiting, diarrhea, malaise and headache. The medication is contraindicated in persons with renal disease. Alternate medications include Famvir (famciclovir, Novartis) and Valtrex (valacyclovir, GlaxoSmithKline), which both offer reduced dosing frequency. In patients who have highly recurrent disease, especially affecting the corneal stroma, 400mg of acyclovir b.i.d. prophylactically for a year or longer significantly reduces recurrence of HSK stromal disease.14
Fungal Infections
Before 2006, fungal keratitis was strongly associated with hot, humid climates and trauma with vegetative material. With last years outbreak of Fusarium keratitis, there has been increased interest in what had been considered a rare form of microbial keratitis. The Fusarium species (figure 3) is the most common cause of keratomycosis (fungal keratitis) in the
Fungal infections have a more insidious presentation than other microbial infections. The usual symptoms of microbial infectionsincluding conjunctival redness, blur, discharge and a corneal disruptionare present, but they are not as acute. Due to the slower, less distinctive progression and relative rarity of this type of keratitis, fungal infections are commonly misdiagnosed as bacterial infections, and treatment is delayed.

3. The Fusarium species is considered a rare form of microbial keratitis, but it is the most common cause of keratomycosis in the United States.
The mainstay topical therapeutic agents have been Natacyn (natamycin 5%, Alcon), which is the only commercially available antifungal, and amphotericin B 0.15%, which must be compounded. Both agents have poor corneal penetration, so consider epithelial scraping or partial debridement to enhance their effectiveness.
A loading dose of an antifungal agent (natamycin alone or alternating natamycin and amphotericin B) during the first one to two hours is recommended (e.g., every 15 minutes) and then every 30 minutes to one hour until the lesion size is notably smaller, indicating that re-epthelialization has begun. Persistent therapy is necessary, as resolution is slow, often taking several weeks. Oral medications, such as ketoconazole, may be added, but no definitive study supports the therapeutic effect of oral treatment.
During the recent occurrences of Fusarium infection, moxifloxacin used as an adjunct therapy demonstrated anti-microbial activity against Candida and Aspergillus speciesbeyond the original design of the medications antibiotic properties.15 Another antifungal, voriconazole (topical and oral), has shown promise in treating Fusarium infections in patients who already underwent penetrating keratoplasty.15
Protozoan Infection
Acanthamoeba is a ubiquitous protozoan organism that feeds primarily on other microbes instead of human tissue. The organism has the ability to encyst when its food supply diminishes, if cell crowding exists or in an environment of reduced pH. All these abilities make treatment of Acanthamoeba infection more difficult.
The case history is essential for diagnosing the patient. Most patients have a history of poor contact lens hygiene and/or exposure to unsanitary water conditions from lakes with elevated temperatures. Exposure may also occur in swimming pools (or hot tubs) with inadequate chemical treatment or municipal water supplies when chlorine levels drop following localized flooding from natural disasters. Indeed, 10% to 15% of patients with Acanthamoeba corneal infection are reported as non-contact lens wearers.16
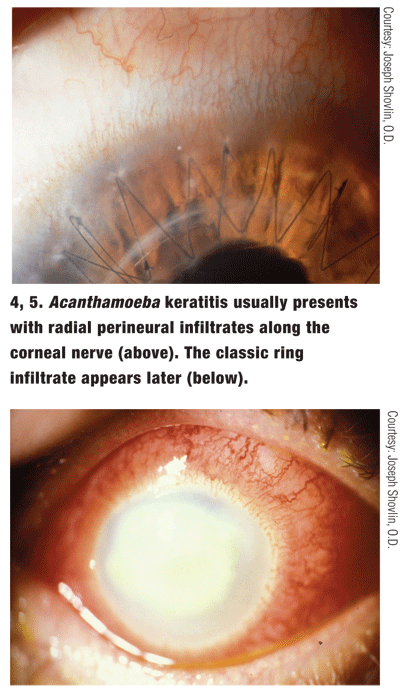
Patients infected with Acanthamoeba typically report unilateral pain that is often out of proportion with the clinical signs, photophobia and tearing. Early presentations of Acanthamoeba keratitis may be similar in appearance to herpetic keratitis; thus, the clinician should be suspicious of dendritic keratitis in a contact lens wearer, especially if the lesion does not respond to antiviral therapy.16 Also consider Acanthamoeba in any contact lens patient who continues to have a progressive keratitis despite antibiotic treatment.
Acanthamoeba keratitis typically starts as a nonspecific keratitis with epithelial micro-erosions or microcystic edema and perineural infiltrates (along the corneal nerves) in a radial pattern (figure 4). The classic ring infiltrate typically appears later in the disease process, with the majority of patients displaying this characteristic after two months (figure 5).17 Other findings associated with Acanthamoeba infection include limbal and scleral inflammation, hypopyon, cataract and glaucoma, especially late in the course of the disease.
MK caused by Acanthamoeba infiltration is very difficult to treat. A timely diagnosis and appropriate treatment, however, can lead to a more favorable outcome.
A broad-spectrum approach is often employed. A combination of Brolene (propamidine isethionate, Sanofi-Aventis), an antimicrobial agent, and polyhexamethylene biguanide (PHMB) is considered the most effective treatment. Brolene, available in 0.1% solution (10ml) and 0.15% ung (5g), is bacteriostatic; it interferes with the chemical processes inside the cell that are required by bacteria, fungi and Acanthamoeba to survive and multiply. Although effective, Brolene produces a significant toxic reaction. PHMB is a cationic disinfectant that has demonstrated high effectiveness in destroying Acanthamoeba (in both cyst and trophozoite forms) at a concentration of 0.02%.18 PHMB is less toxic to the cornea than other treatments.
When using these medications, dosing is usually started at every one to two hours until improvement occurs, which may take one to two weeks. At that point, dosage is reduced but may last for three to four months after inflammation has resolved.16
Other medications that have been used to treat corneal Acanthamoeba infections, in combination with those mentioned, are topical neomycin, clotrimazole and chlorhexidine.
Any condition that results in significant corneal compromise can lead to inflammation of adjacent tissues, including the anterior uvea. A cycloplegic agent, such as homatropine 5% t.i.d., is recommended in all cases of MK except where otherwise contraindicated. The cycloplegic agent has three functions: It puts the iris and ciliary body at rest, meaning less patient discomfort. It also moves the pupil away from the apex of the lens, making posterior synechiae less likely. Finally, it partially reduces the vascular permeability of the anterior uveal blood vessels, which in turn, reduces the severity of the anterior chamber reaction.
A greater challenge when treating MK is deciding whether a topical corticosteroid is needed to decrease the risk of immune-mediated damage to the cornea. Historically, topical corticosteroids have been used only after complete re-epithelialization of infectious ulcers. This practice is still the standard of care when treating infections caused by HSV, fungi and protozoa. Because antimicrobial agents available against these organisms are not 100% effective, the patients immune system is required to assist in the battle against these bugs.
Early introduction of steroids appears to result in a more favorable outcome to eyes that display significant corneal inflammation secondary to bacterial keratitis. So, corticosteroid therapy is often begun as soon as the selected antimicrobial therapy is seen to be effective, 24 to 72 hours after the antibiotic is initiated. A typical dosing pattern for a corticosteroid is Pred Forte (prednisolone acetate 1%, Allergan) q1h to q.i.d.
In more peripheral lesions in which there is no significant inflammatory response, steroids may be unnecessary. To help answer questions regarding the timing and indications for topical steroid management of corneal ulcers, the National Eye Institute is funding the Steroids for Corneal Ulcers Trial (SCUT), to be conducted in India.19 The study has not yet begun, although a pilot study of 42 patients showed better visual acuity and smaller infiltrate/scar size but longer re-epithelialization time with steroidal treatment.
Any time a lesion is not resolving as expected with the chosen antimicrobial agent, you need to reconsider your diagnosis before adding a steroid. Recall that fungal infections and Acanthamoeba keratitis are often initially misdiagnosed, in part because the epithelium may be intact in some of these cases; adding a steroid, which would reduce the contribution of the patients immune system, would be dangerous with these infections.
When evaluating a patient with suspected MK, a careful history and review all of the patients signs and symptoms are critical. Awareness of characteristic ocular findings and the epidemiology of pathogens is invaluable. While various treatment options may be beneficial in the care of a patient with an infectious keratitis, there is little doubt that proper identification of the specific etiology early on will result in the best visual prognosis for the patient.
Drs. Black and Tyler are assistant professors at Nova Southeastern University College of Optometry. Dr. Black is also the director of the Broward Boulevard Eye Clinic. Dr. Tyler is also the module two director of primary care at the Davie campus. Dr. Reed is an associate professor at Nova Southeastern University College of Optometry, as well as the director of externships.
1. Cokington CD, Hyndiuk RA. Bacterial Keratitis. In: Tabbara KF, Hyndiuk RA (eds). Infections of the Eye. 2nd ed. Little, Brown &
2. Keay L, Edwards K, Naduvilath T, Taylor HR, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology 2006 Jan;113(1):109-16.
3. Matsumoto K, Ikema K,Tanihara H. Role of cytokines and chemokines in pseudomonal keratitis. Cornea 2005 Nov;24(8 Suppl):S43-S49.24(S1):S43-9.
4. McLeod SD. The role of cultures in the management of ulcerative keratitis. Cornea1997 Jul;16(4):381-2.
5. Marangon FB, Miller D, Alfonso EC. Impact of prior therapy on the recovery and frequency of corneal pathogens. Cornea 2004 Mar;23(2):158-64.
6. Kunimoto DY, Sharma S, Garg P, Rao GN. In vitro susceptibility of bacterial keratitis pathogens to ciprofloxacin. Emerging resistance. Ophthalmology 1999 Jan;106(1):80-5.
7. Mah-Sadorra JH, Najjar DM, Rapuano CJ, Laibson PR, et al. Serratia cornal ulcers a retrospective clinical study. Cornea 2005 Oct;24(7):793-800.
8. Garg P, Sharma S, Rao, G. Ciprofloxacin-resistant Pseudomonas keratitis. Opthal 1999;106 (7):1319-23.
9. Mah-Sadorra JH, Yavuz SG, Najjar DM, et al. Trends in contact lens-related corneal ulcers. Cornea 2005 Jan;24(1):51-58.
10. Kowalski RP, Karenchak LM, Romanowski EG. Infectious disease: changing antibiotic susceptibility. Ophthalmol Clin North Amer 2003 Mar;16(1):1-9.
11. Kowalski RP, Pandya AN, Karenchak LM, Romanowski EG, et al. An in vitro resistance study of levofloxacin, ciprofloxacin, and ofloxacin using keratitis isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Ophthalmology 2001 Oct;108(10):1826-9.
12. Solomon R, Donnenfeld ED, Perry HD, et al. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology 2005 Mar;112(3):466-9.
13. McElvanney A. Doxycycline in the management of Pseudomonas corneal melting: two case reports and a review of the literature. Eye & Contact Lens 2003 Oct;29(4):258-61.
14. Sudesh S, Laibson PR. The impact of the herpetic eye disease studies on the management of herpes simplex virus ocular infections. Curr Opin Ophthalmol 1999 Aug;10(4):230-3.
15. Klont RR, Eggink CA, Rijs AJ, Wesseling P, et al. Successful treatment of Fusarium keratitis with cornea transplantation and topical and systemic voriconazole. Clin Infect Dis 2005 Jun 15;40(12):110-2.
16. Illingworth CD, Cook SD. Acanthamoeba keratitis. Surv Ophthalmol 1998 May-Jun;42(6):493-508.
17. Bacon AS, Frazer DG, Dart JK, Matheson M, et al. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984-1992. Eye 1993;7(Pt. 6):719-25.
18. Larkin DFP, Kilvington S, Dart JK. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology 1992 Feb;99(2):185-191.
19. National Eye Institute. Steroids for Corneal Ulcers Trial. Available at: http://www.nei.nih.
gov/neitrials/viewStudyWeb.aspx?id=121. (Accessed December 19, 2006)

