 |
A 45-year-old female reported to the office for a six-month posterior segment follow up without complaints. Her acuity was good, there was no history of glaucoma or blunt trauma, she was neither diabetic nor hypertensive and reported no allergies of any kind. She had an ocular history of a posterior segment finding requiring monitoring (seen in the photograph below).
Diagnostic Data
The patient’s best-corrected visual acuities were 20/20 at distance and near in each eye. Her external examination was normal and there was no afferent pupillary defect. Her confrontation fields were full. Refraction demonstrated negligible changes. Biomicroscopy examination found normal anterior segment structures with open angles (by Van Herick technique) and normal applanation pressures of 16mm Hg OU. The only concern was the posterior segment finding.
Additional studies included OCT to further investigate the status of the retinal and subretinal tissues and their relationship to Bruch’s membrane and the retinal pigment epithelium (RPE), and OCT angiography (OCT-A) to examine the subretinal/choroidal tissues for choroidal neovascular membrane formation. In addition to the above, a sodium fluorescein angiogram may also be indicated in this case to rule out vascular support, and fundus autofluorescence could be considered to identify the presence or absence of drusen.
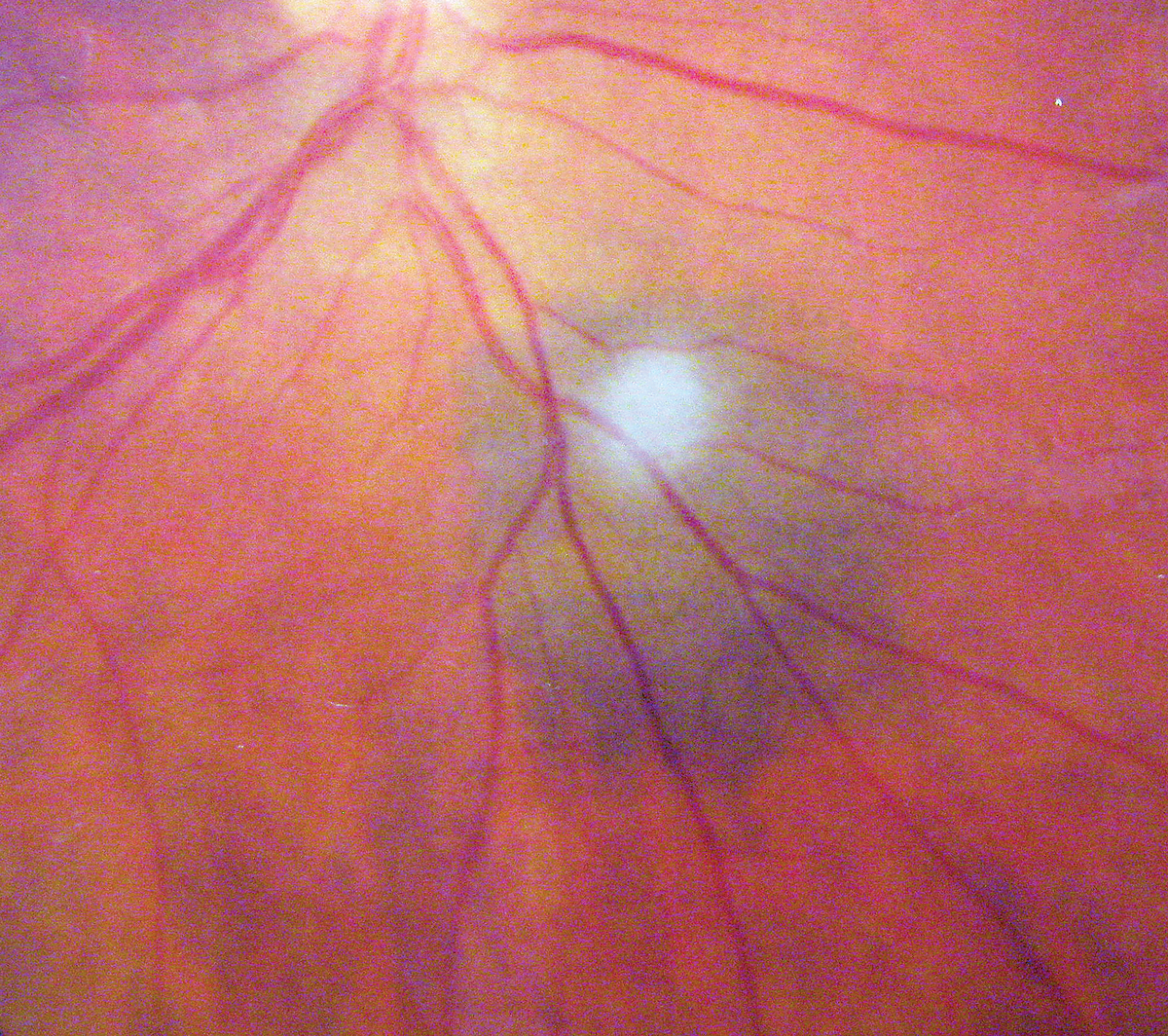 |
|
What do these findings suggest about the patient? How would you approach management? Click image to enlarge. |
Presentation
The diagnosis in this issue is choroidal nevus. Both choroidal nevi and choroidal melanomas represent space-occupying masses of the uveal tract. Choroidal nevi appear as round or oval, flat or slightly elevated (1mm or less) lesions within the posterior fundus. Their margins are typically detectable but indistinct.1
Nevi present with a variety of hues, but most commonly they appear slate-blue or greenish-gray in coloration.1 There may be overlying areas of drusen noted as well, indicating longstanding presence.1,2 The vast majority of choroidal nevi remain under two disc diameters (DD) or 3mm in size.1 Larger nevi, particularly those in excess of 4DD/6mm, carry increased suspicion of malignancy.3,4 Generally, patients with choroidal nevi are asymptomatic, with the lesion detected upon indirect binocular or biomicroscopic ophthalmoscopy.
Choroidal melanomas, in contrast to nevi, appear as mottled, often dome-shaped lesions of the ocular fundus. Coloration varies widely, ranging from complete amelanosis (i.e., white) to jet-black. Most commonly, lesions are a non-uniform greenish-gray.4,5 There may be significant elevation. As they grow, melanomas may break through Bruch’s membrane, taking on a “mushroom-shaped” appearance. Serous retinal detachments are commonly associated with this presentation.4-6 Overlying orange pigmentation known as lipofuscin may also be seen, which is considered by many to be a pathognomonic sign of malignancy.7
Many patients with choroidal melanomas are entirely asymptomatic; in most cases, lesions are detected via dilated, indirect ophthalmoscopy on routine optometric visits. However, larger lesions or those in close proximity to the macula may induce visual symptoms that prompt the patient to seek examination. These symptoms may include photopsia, visual field deficit, metamorphopsia or decreased acuity secondary to subretinal fluid accumulation and/or hyperopic refractive shift.4
The vast majority of patients with choroidal melanoma are over the age of 50, though the tumor may rarely occur in childhood.8,9 Race also plays a significant role in the distribution of choroidal melanoma. Caucasians are perhaps three times more likely than Asians to manifest choroidal melanoma and at least eight times more likely than those of African descent.10-12 Not surprisingly, patients with light-colored irides (e.g., blue or grey) also seem to be at greater risk for developing uveal melanomas.13 The presence of numerous cutaneous nevi—particularly dysplastic nevi—is yet another risk factor.13,14
Pathophysiology
Choroidal nevi and melanomas are both derived from uveal melanocytes. In the mid-1960s, Naumann et al. identified the four atypical cell types inherent in choroidal nevi; in order of prevalence, these include: plump polyhedral cells, slender spindle cells, intermediate cells and balloon cells.15 In contrast, melanomas are comprised of malignant melanocytes. The Callendar classification system for choroidal melanomas suggests that there are also four types of cells in these lesions: spindle A, spindle B, fascicular and epithelioid.16,17 In general, the presence of epithelioid cells within a melanoma heralds a poorer prognosis.18
There is some controversy regarding the precise pathogenesis of melanomas. It is believed that nevi may convert to malignancy in a small percentage of individuals; historically, the annual rate of malignant transformation is about one in five thousand cases, though a more recent study suggests it is lower—perhaps one in 8,845.19,20 Risk factors for malignant transformation of nevi include diameter (>5mm), thickness (>1mm), the presence of lipofuscin and serous retinal detachment.1
Ultraviolet (UV) radiation has also been associated with the development of ocular and non-ocular melanoma.4 However, the specific role of UV in the development of choroidal melanoma is uncertain. While some studies indicate a causal relationship, others are less conclusive.21-25 Current opinion holds that UV radiation is a controversial factor in the pathogenesis of choroidal melanoma.
While some choroidal nevi possess the capacity for malignant growth, the majority are completely benign and require only periodic monitoring. Of course, differentiating between a large, atypical nevus and a small choroidal melanoma requires experience and expertise. Ancillary procedures that may facilitate an accurate diagnosis include stereo photography, standardized ultrasonography, fluorescein angiography and optical coherence tomography (OCT).26 More invasive procedures, including transvitreal fine-needle aspiration biopsy, are also used to differentiate suspicious lesions.27
Protocols
Those patients diagnosed with choroidal melanoma should be referred for prompt medical evaluation by an internist and/or an oncologist. Specifically, this is done to ascertain whether there are any additional primary or metastatic malignancies present. The systemic work-up should include a thorough medical and family history, physical examination and directed laboratory evaluation.
Therapy for choroidal melanoma has changed radically in the last 30 years. Until the late 1970s, enucleation was considered the only definitive treatment and the best option for survival among those with melanoma. In 1978, however, a pivotal paper by Zimmerman et al. challenged conventional thinking, suggesting that enucleation might actually contribute to systemic metastasis.28 This article and another subsequent publication provided the impetus to develop alternative therapies for choroidal melanoma, most notably radiotherapy and tumor resection.29
Today, therapy for choroidal melanoma is dictated primarily by the size of the lesion. The Collaborative Ocular Melanoma Study (COMS) defined tumors as small (5mm to 16mm in basal diameter and 1.0mm to 2.5mm in height), medium (<16mm diameter and 2.5mm to 10.0mm in height), or large (>16mm diameter and/or >10mm in height).30 Small tumors may be treated by simple observation, but therapy is initiated if any sign of growth or visual compromise is encountered.
Focal laser photocoagulation and, more recently, transpupillary thermotherapy, have been employed successfully for selected small melanomas.31,32 For some small melanomas, as well as the majority of medium-sized choroidal melanomas, radiation remains the treatment of first choice.31 Brachytherapy—in which a plaque with embedded radioactive material is temporarily sutured to the episclera overlying the tumor—is the most common method used today. Another method, employing charged particle irradiation (also known as external beam irradiation), may also be employed for certain tumors. Overall, the success rates and complications (including radiation retinopathy and cataract formation) for plaque therapy and external beam therapy are similar. However, since external beam irradiation does not require surgery, it may be preferred in some cases.
Another treatment option for medium-sized tumors is local resection using a partial lamellar sclerouvectomy technique.33 This procedure, when successful, offers significant advantages over radiation therapy. However, the technique is quite challenging even for the most experienced of surgeons and carries with it significant risks for vitreous hemorrhage, retinal detachment, and cataractogenesis. Local resection of choroidal melanoma is preferred for smaller, more anteriorly located tumors.
Despite controversy, enucleation is still used for the treatment of some uveal melanomas. Generally, enucleation is reserved for advanced melanomas that: (a) occupy most of the intraocular space, (b) have induced a secondary glaucoma or (c) have invaded the optic nerve. When enucleation is performed on an eye with melanoma, care is taken not to clamp the optic nerve or aggressively handle the eye, in an effort to reduce potential tumor seeding and metastasis.31 For those advanced tumors that demonstrate massive extrascleral extension into the orbit, and in which the eye is blind and painful, eyelid-sparing orbital exenteration is typically justified.34
Prognosis
The etymology of the term “choroidal melanoma” is somewhat interesting. Historically, scientists defined a non-malignant accumulation of melanocytes—that which we refer to today as a choroidal nevus—as a benign choroidal melanoma. As recently as 1994, Alexander’s Primary Care of the Posterior Segment (2nd Edition, McGraw-Hill) continued to use this nomenclature. To avoid misinterpretation and unnecessary emotional stress for patients, it is perhaps best to use the terms “nevus” and “melanoma” to refer to benign and malignant choroidal lesions, respectively. While the majority of choroidal melanomas occur in older, white individuals, younger patients and those of African or Asian descent are not immune.
Choroidal melanoma presents a potentially life-threatening situation because of its propensity toward metastasis. These tumors have been known to spread to numerous organ systems including the liver, lungs, skin, and gastrointestinal tract. In addition to ocular therapy, patients with newly detected melanomas should be referred for laboratory testing, which may include hematology, chest CT and liver function studies among others. The liver is a particular area of interest, since it represents the primary site of metastasis for uveal melanoma.4
This patient was referred to Wills Eye Hospital, where it was determined the lesion was not malignant but requiring close monitoring.
Dr. Gurwood thanks Dr. Alan Kabat for his contributions to this case.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
1. Sumich P, Mitchell P, Wang JJ. Choroidal nevi in a white population: the Blue Mountains Eye Study. Arch Ophthalmol 1998; 116(5):645-50. 2. Shields CL, Mashayekhi A, Materin MA, et al. Optical coherence tomography of choroidal nevus in 120 patients. Retina 2005; 25(3):243-52. 3. Butler P, Char DH, Zarbin M, et al. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology 1994;101(4):710-6. 4. Char DH. Ocular melanoma. Surg Clin North Am 2003; 83(2):253-74. 5. Ou JI, Wheeler SM, O'Brien JM. Posterior pole tumor update. Ophthalmol Clin North Am 2002; 15(4):489-501. 6. Kalkman E, Baxter G. Melanoma. Clin Radiol 2004; 59(4):313-26. 7. Shields JA, Shields CL, Donoso LA. Management of posterior uveal melanoma. Surv Ophthalmol 1991; 36(3):161-95. 8. Grin JM, Grant-Kels JM, Grin CM, et al. Ocular melanomas and melanocytic lesions of the eye. J Am Acad Dermatol 1998;38(5 Pt 1): 716-30. 9. Stanford DG, Hart R, Thompson JF. Ocular melanoma in childhood. Aust N Z J Surg 1993;63(9):729-31. 10. Biswas J, Krishnakumar S, Shanmugam MP. Uveal melanoma in Asian Indians: a clinicopathological study. Arch Ophthalmol 2002; 120(4):522-3. 11. Egan KM, Seddon JM, Glynn RJ, et al. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol 1988; 32(4):239– 51. 12. Neugut AI, Kizelnik-Freilich S, Ackerman C. Black-white differences in risk for cutaneous, ocular and visceral melanomas. Am J Public Health 1994; 84(11):1828-9. 13. Vajdic CM, Kricker A, Giblin M, et al. Eye color and cutaneous nevi predict risk of ocular melanoma in Australia. Int J Cancer 2001; 92(6):906–12 14. Hammer H, Olah J, Toth-Molnar E. Dysplastic nevi are a risk factor for uveal melanoma. Eur J Ophthalmol 1996; 6(4):472-4. 15. Naumann G, Yanoff M, Zimmerman LE. Histogenesis of malignant melanomas of the uvea: I. Histopathologic characteristics of nevi of the choroid and ciliary body. Arch Ophthalmol 1966; 76(6): 784-96. 16. Callender GR. Malignant melanotic tumors of the eye: a study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolaryngol 1931; 36:131-42. 17. McLean IW, Foster WD, Zimmerman LE, Gamel JW. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol 1983; 96(4):502-9. 18. Augsburger JJ, Gonder JR, Amsel J, et al. Growth rates and doubling times of posterior uveal melanomas. Ophthalmology 1984; 91(12):1709–15. 19. Ganley JP, Comstock GW. Benign nevi and malignant melanomas of the choroid. Am J Ophthalmol 1973;76(1):19–25. 20. Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005; 112(10):1784-9. 21. Tucker MA, Shields JA, Hartge P, et al. Sunlight exposure as risk factor for intraocular malignant melanoma. N Engl J Med 1985; 313(13):789-92. 22. Lutz JM, Cree IA, Foss AJE. Risk factors for intraocular melanoma and occupational exposure. Br J Ophthalmol 1999;83(10):1190-3. 23. Holly EA, Aston DA, Char DH, et al. Uveal melanoma in relation to ultraviolet light exposure and host factors. Cancer Res 1990; 50(18):5773-7. 24. Seddon JM, Gragoudas ES, Glynn RJ, et al. Host factors, UV radiation, and risk of uveal melanoma: a case-control study. Arch Ophthalmol 1990; 108(9):1274-80. 25. Schwartz LH, Ferrand R, Boelle PY, et al. Lack of correlation between the location of choroidal melanoma and ultraviolet-radiation dose distribution. Radiat Res 1997; 147(4):451-6. 26. Muscat S, Parks S, Kemp E, Keating D. Secondary retinal changes associated with choroidal naevi and melanomas documented by optical coherence tomography. Br J Ophthalmol 2004; 88(1):120-4. 27. Augsburger JJ, Correa ZM, Schneider S, et al. Diagnostic transvitreal fine-needle aspiration biopsy of small melanocytic choroidal tumors in nevus versus melanoma category. Trans Am Ophthalmol Soc; 100:225-32 28. Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br J Ophthalmol 1978; 62(6):420-5. 29. Zimmerman LE, McLean IW. An evaluation of enucleation in the management of uveal melanomas. Am J Ophthalmol 1979; 87():741-60. 30. Singh AD, Kivela T. The collaborative ocular melanoma study. Ophthalmol Clin North Am 2005; 18(1):129-42. 31. Shields CL, Shields JA. Recent developments in the management of choroidal melanoma. 32. Shields CL, Shields JA, Perez N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology 2002; 109(2):225-34. 33. Shields JA, Shields CL, Shah P, et al. Partial lamellar sclerouvectomy for ciliary body and choroidal tumors. Ophthalmology 1991; 98(6):971-83. 34. Shields JA, Shields CL, Demirci H, et al. Experience with eyelid-sparing orbital exenteration. The 2000 Tullos O. Coston Lecture. Ophthal Plast Reconstr Surg 2001; 17(5):355-61. 35. Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. III. Initial mortality findings. COMS report no. 18. Arch Ophthalmol 2001; 119(7):969-82. 36. Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma. II. Initial mortality findings. COMS report no. 10. Am J Ophthalmol 1998; 125(6):779-96. |

