Annual Innovations in Eye Care IssueFollow the links below to read other articles from our annual Innovations in Eye Care issue: Breaking the Burden: A New Way to Deliver Anti-VEGF Prospects for Neuroprotection in Glaucoma |
The ability to design technology that can mimic some elements of human cognition, once purely the stuff of science fiction, is becoming a reality.1 Medicine generally and eye care specifically are among the facets of daily life that stand to be transformed by this technology, allowing doctors to become more accessible and efficient.2 The successful optometric practice of the future will be one that chooses to see artificial intelligence (AI) not as a threat but rather as a tool to enhance uniquely human skills such as intuition and insight that are the hallmark of a good doctor.
“Medicine begins where the technology ends,” bioethicist Edmund Pellegrino said 30 years ago, and it remains true today.3
But how will this disruptive technology evolve, and where will it fit into modern inter-professional health care teams? No doubt AI will affect many aspects of care, but the first frontier will be our diabetes patients. In fact, it’s happening already. Efforts are underway in the United Kingdom, India, Australia and Silicon Valley, among other sites. Interestingly, at least two projects involve teen prodigies partnering with medical and computer science pros.
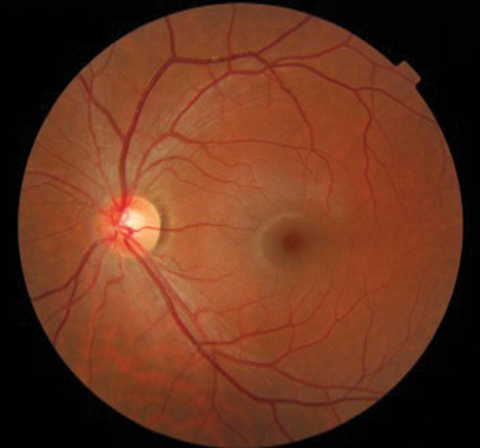 |
| Deep learning software can help AI systems identify the difference between a normal retina, as seen here, and one with signs of diabetic retinopathy. |
Tech-assisted Diagnosis
Diabetic retinopathy (DR) screening may be the optometric clinical responsibility best suited for augmentation by AI, given certain inescapable facts about the population affected and their access to care—or lack thereof.
For one, the prevalence of diabetes is increasing. Epidemiologically, the worldwide diabetes population grew from 153 million in 1980 to 347 million in 2008, and 25.6 million, or 11.3%, of the US population in 2010 had diabetes.4 Estimates show that nearly 86% of Type 1 diabetes mellitus (DM) patients and 40% of Type 2 DM patients have DR.5 Furthermore, a high association exists between disease duration and loss of vision.
Despite these numbers, such patients are woefully underserved in the current health care system. Research estimates more than one-third of all adult diabetes cases in the United States remain undiagnosed.6 And an American Optometric Association survey found that four out of five Americans don’t realize that diabetic eye disease has no visible symptoms in the early stages.7 Lack of awareness and difficulty accessing routine eye care leads many patients to remain undiagnosed until later in the course of the disease.
Technology has already made small improvements to the care of patients with DR. Digital imaging makes serial evaluation of images easier and more collaborative. Computer-navigated surgical tools improve the safety of laser surgery for proliferative DR.8 And at the 2017 Association for Research in Vision and Ophthalmology meeting, surgeons demonstrated the first successful use of a remote-controlled robotic system in the human eye during retinal surgery, with fewer complications in the robot-assisted group compared with the standard manual approach.9
But the truly revolutionary frontier lies in efforts to develop software that can screen at-risk patients and identify the earliest signs of disease. Successes in cardiology may give us a sense of what’s in store for eye care. Computer scientists at Stanford University have developed algorithms that can diagnose heart arrhythmias from electrocardiography signals. The algorithm is cardiologist-level accurate, not subject to fatigue and can detect arrhythmias continuously with real-time efficiency.10 The technology, placed in a device at-risk patients wear at all times, can alert emergency services to serious heartbeat irregularities as they happen.
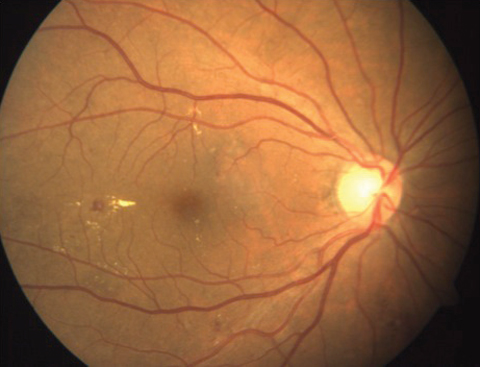
| These new tools may one day provide screening services to underserved populations, giving doctors the chance to catch signs of advancing disease much earlier and avoid severe non-proliferative DR, as seen here. |
Deep Thoughts on DR
The essence of computer-based health screening is reducing fundamental diagnostic dilemmas to mere data analysis problems. In DR research, AI software is fed a huge database of images and trained to distinguish between healthy and DR-affected eyes by teaching it to recognize the characteristics of each.
While the ability to compare images to normative databases and identify outliers has been a feature of modern medicine for years, newer software algorithms employ so-called deep learning, in which analysis takes place incrementally at multiple levels, beginning at the most rudimentary and progressing with ever-increasing sophistication.
In image detection, AI software may begin by assessing just a few pixels from a tiny section of the image, noting those relationships and assigning a weight or likelihood that the image meets the target identity; in this case, diabetic retinopathy. It then polls the next level of analysis (for instance, advancing from pixels to simple shapes) to see if it too fits the pattern in question, then moves up another level, and so on. Eventually, a confluence of validating findings arises among multiple levels and the image is classified.11 Along the way, the software learns which associations are seen most often and applies that new knowledge to the next scan. It is this self-improving capability that earns it the labels machine learning or artificial intelligence. The more layers of abstraction in the algorithm, the “deeper” the learning—and the more accurate the result.
In one retrospective study, a deep-learning software algorithm analyzed a database of 1,748 retinal fundus photos from 874 diabetes patients. Results showed 96.8% sensitivity and 87% specificity for DR, which represented a 30% greater specificity than software analysis that lacked deep learning.12
Another project used 54 ophthalmologists to evaluate 128,175 retinal images for the presence of DR and diabetic macular edema, as well as image quality.11 The DR severity (none, mild, moderate, severe or proliferative) was graded according to the International Clinical Diabetic Retinopathy scale. An AI algorithm was designed to identify ‘referable’ cases—defined as moderate or worse DR—as a way to demonstrate its viability as a screening tool. Its performance in several tests yielded sensitivities for referable DR that ranged from 87% to 97.5%, and specificity of 94%. The study authors conclude, “these results demonstrate that deep neural networks can be trained, using large data sets and without having to specify lesion-based features, to identify diabetic retinopathy or diabetic macular edema in retinal fundus images with high sensitivity and high specificity.” They also note that an algorithm’s sensitivity and specificity “can be tuned to match requirements for specific clinical settings, such as high sensitivity for a screening setting.”11
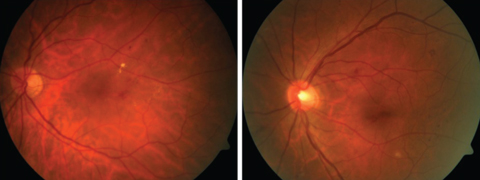 |
| By teaching AI software to distinguish from mild (left) and moderate (right) non-proliferative DR, researchers demonstrated an AI algorithm’s viability as a screening tool. The system identified ‘referable’ cases—moderate or worse DR—with sensitivities ranging from 87% to 97.5%, and specificity of 94%.11 Click images to enlarge. |
AI in the Exam Room
Several groups have met with success in modeling deep-learning AI to DR screening. Players range from big fish in computer science to high school students. It’s too soon to tell which, if any, of these efforts will be commercialized and widely accepted by medical professionals, but here’s a look at early movers in AI for DR.
• Google’s DeepMind. London-based AI firm DeepMind, acquired by Google in 2014, is working with Moorfields Eye Hospital to develop algorithms for early detection of DR and age-related macular degeneration. The team is reviewing fundus photos and optical coherence tomography (OCT) scans from more than one million exams that took place at Moorfields in 2017.13 In addition to imaging, the dataset will also include each patient’s demographic information, disease status, treatment history, imaging devices used and time elapsed between visits. By combining imaging with exam data, the team is exploring not just automated grading of fundus photos and OCT scans, as in other AI efforts, but also the feasibility of developing “novel quantitative measures for specific disease features and for monitoring the therapeutic success.”13 The work is ongoing and results have yet to be announced.
• EyeLogic. A smartphone app now under development, this uses an algorithm built from analysis of more than 75,000 fundus images found in the Eye Picture Archive Communication System (EyePACS) telemedicine database. Published results show 94% sensitivity and 98% specificity for diabetic retinopathy.14 Given that the technology identified cases that need further evaluation and treatment with high reliability, the authors conclude that a fully data-driven AI-based grading algorithm has the potential to be used to screen fundus appearance in patients with diabetes.
Created by 18-year-old Rishab Gareya of San Jose, Calif., the work earned him a $50,000 scholarship to Stanford.
• Eyeagnosis. A second teen techie, 16-year-old Kavya Kopparapu of Herndon, Va., created an app that uses a 3D-printed lens as a smartphone accessory to enable self-administered eye exams. The AI was designed using off-the-shelf Microsoft code and a database of 34,000 retina scans from the National Institutes of Health.15
Tests performed at a hospital in Mumbai found the app can “spot diabetic retinopathy with the accuracy of a human pathologist,” according to IEEE Spectrum.15 It also can identify retinal microaneurysms. The app remains in testing.
• Dr. Grader. Australia’s Commonwealth Scientific and Industrial Research Organization (CSIRO) developed this AI platform and is about to deploy it at 20 general practitioners’ (GP) offices in Western Australia. The software was built from an IBM database of 88,000 retinal images in the EyePACS library. At-risk patients “would usually be referred to a specialist for screening, waiting six weeks or more—now it can potentially be done in a single 30-minute visit to a GP,” according to CSIRO.16 If successful, the app could help reduce the $14 billion annual impact of diabetes on the Australian economy, CSIRO says.
• Optos/Verily partnership. In January 2017, another Google company, Verily Life Science, announced a partnership with Nikon and its subsidiary Optos to create new DR screening protocols.17 No other details have been released, but the effort is expected to combine Verily’s machine learning technology with Optos’ ultra-widefield imaging already popular in today’s practices.
 |
| As AI technology for DR progresses, researchers hope it will one day provide screening advanced enough to properly identify any and all disease stages, even borderline cases, such as mild/moderate non-proliferative DR, as seen here. |
The Future in Focus
The team at DeepMind note several ways AI might reduce the worldwide loss of vision from diabetic retinopathy:18 Screening tests, either self-administered with a mobile device or used in telemedicine networks with medical professionals, can extend access to care for underserved populations. Subclinical disease may be identified early enough to allow initiation of treatment and lifestyle modifications to forestall damage in ways never before possible. And since these new software platforms are learning engines, it’s reasonable to expect continued refinement of accuracy and capability.
As artificial intelligence and other technologies evolve, optometry will have opportunities to bring patients into the healthcare system earlier than before and make meaningful differences in their lives. With these innovative technologies, optometrists may one day be able to improve patient outcomes, positively impact optometric education and create new models of inter-professional care. For us to continue to positively impact the many diverse communities that optometry serves, the future starts now.
Dr. Wong is the director of New Technologies, director of Clinical Externships and the former chief of Adult and Pediatric Primary Eye Care at the SUNY College of Optometry.
Dr. Steinway is an assistant clinical professor in the Primary Eye Care Service at the SUNY College of Optometry.
Dr. Poirier is an assistant clinical professor in the Advanced Eye Care Service at the SUNY College of Optometry.
Dr. Gould is the chief of the advanced Care Service at the SUNY College of Optometry.
1. DesMarais C. Elon Musk is right, artificial intelligence is growing like crazy. November 29, 2017. www.inc.com/christina-desmarais/elon-musk-is-right-artificial-intelligence-is-growing-like-crazy.html. Accessed January 25, 2018. |

