The pace of technological advancement has granted optometrists a more targeted, more robust and more comprehensive ability to diagnose, evaluate and treat a plethora of ocular pathologies. Specifically, new retinal devices may supplant or enhance their technological predecessors, all of which serve to benefit optometrists and patients alike.
Where ODs once had to rely on sometimes invasive techniques, we now have non-invasive options. Ocular examinations that previously required a considerable amount of time can now be done instantly. Some diagnostic and monitoring procedures that had in years past required comanagement with ophthalmology are now in the hands optometrists alone.
Technology isn’t only changing what optometrists can accomplish; it’s changing what optometry is.
This article delves into the nuances and benefits of the machines that are guiding the way, the traditional techniques ODs still must master and how a combination of the old and the new can be used in the modern clinic.
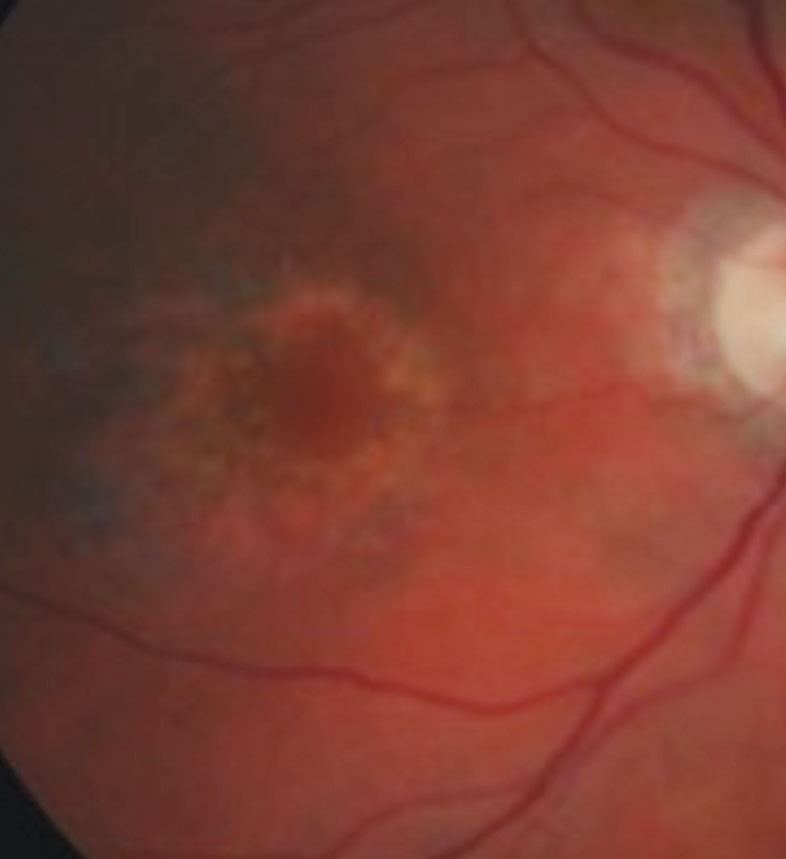 |
| This photo of a dilated fundus exam portrays a patient with parafoveal ring-shaped “bull’s eye” retinal pigment epithelium defects secondary to Plaquenil toxicity. Photo: Marlon Demeritt, OD. |
Dilated Fundus Exam vs. Widefield Retinal Imaging
The one practice that predates every mode of technology is a dilated fundus examination. A dilated exam remains the standard practice, and its use is not overridden by the development of new technologies. But that doesn’t mean it’s perfect. This traditional method expands the ability to view the retina in its entirety and allows for a better appreciation of the depth and contour of many clinical entities. However, dilation can take approximately 15 minutes to take effect and can last anywhere from two to four hours, rendering the patient temporarily light sensitive and unable to see up close.1 Some patients may even experience “tightness” or “heaviness” of their eyelids, which may serve to hinder their ability to drive immediately following their eye examination.1 According to the National Eye Institute, it is recommended to have a dilated eye exam annually after the age of 40, which also means experiencing these side effects on an annual basis.1,2
Nevertheless, dilated eye exams play an integral role in delineating potential problems that imaging devices may flag. Many retinal imaging modalities may produce false positives and false negatives that can skew our management decisions in the wrong direction. As such, dilation allows for further, accurate analysis that will yield the best possible ocular and systemic outcomes for all patients alike.
As a supplement to dilated exams, ultra-widefield high-resolution retinal imaging can non-invasively help to evaluate, diagnose and document the peripheral retina and may also capture peripheral retinal pathology, such as capillary non-perfusion, neovascularization and retinal detachments, that may otherwise go undetected through traditional techniques. The technology is equipped with multiple imaging options including color, red-free, choroidal and autofluorescence features.2,3 Imaging views can also be modified to include standard 200˚ single capture, auto montage and stereo imaging.3
These imaging devices serve to benefit the clinical practice and patients in several ways. To begin, it can be used as a visual aid that can facilitate treatment and management discussions, both of which serve to increase patient compliance. One study suggests that widefield imaging may be 30% better at locating peripheral retinal lesions such as holes, tears, nevi and hemorrhages due to its ability to provide 200-degree temporal and nasal imaging.2,3
In addition, widefield imaging allows for visual documentation. This can track the progression or resolution of retinal abnormalities, which can crucially affect management protocol.
Finally, widefield imaging is time-efficient as it can be used to screen the retina for possible abnormalities, which can be further analyzed through traditional dilating techniques.
Caveats to widefield retinal imaging include patient cooperation, possible artifacts, training needed to capture good retinal images and poor quality of images due to media obstruction.3 Additionally, this form of imaging, while convenient and useful, may give patients the idea that it surpasses or even replaces dilated exams. With that said, if optometrists are going to offer this service, it’s vital to explain to patients that these images are used to supplement rather than replace dilated fundus examinations.
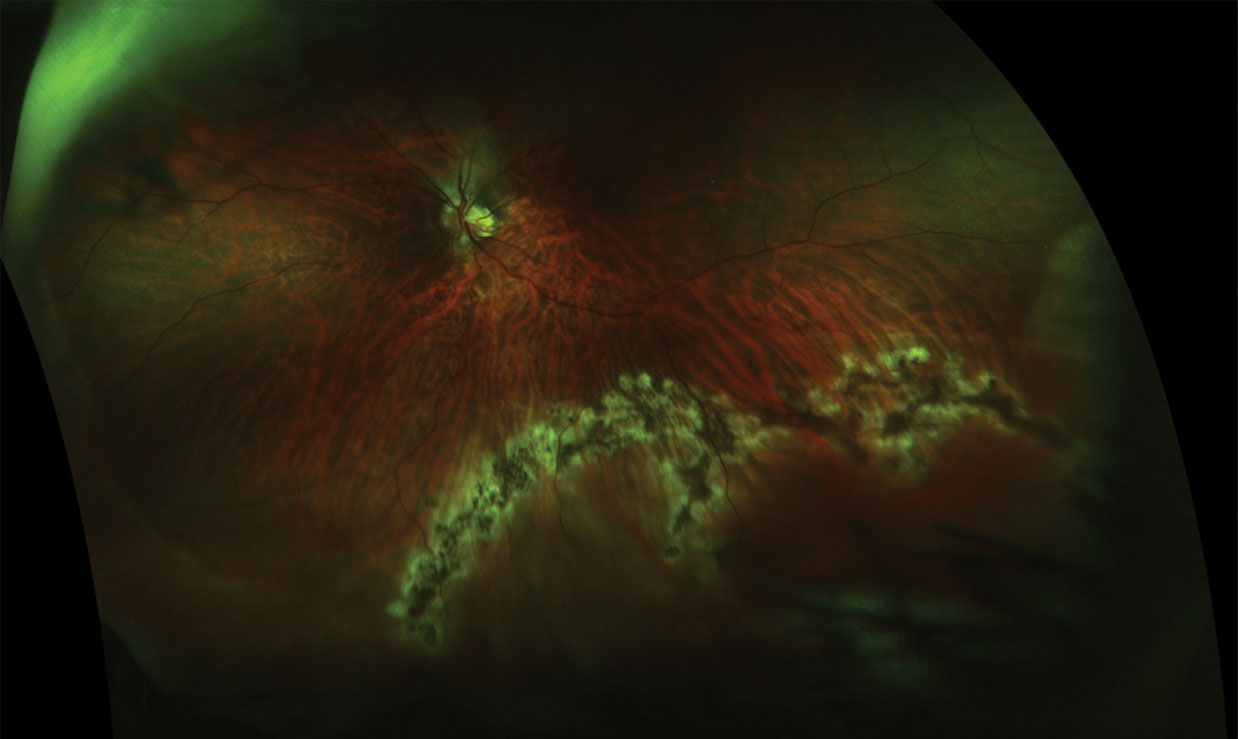 |
| This retinal break with retinal detachment, seen post-treatment (above), was diagnosed using dilated funduscopy, but was not apparent pre-treatment (below) using ultra-widefield imaging. Click images to enlarge. Photos: Jessica Steen, OD. 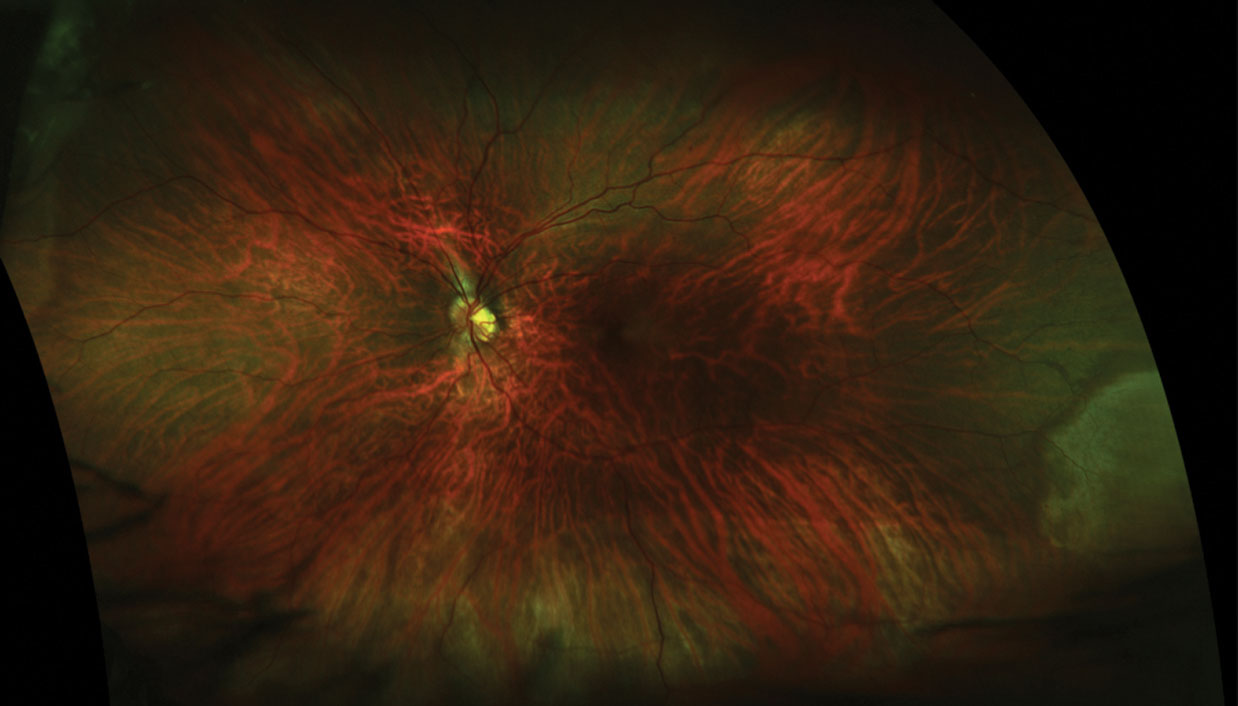 |
Slit Lamp Indirect Ophthalmoscopy vs. Optical Coherence Tomography
Slit lamp indirect ophthalmoscopy is another facet of a complete dilated fundus examination. With the use of high-plus power lenses, such as 78D or 90D lenses, indirect ophthalmoscopy at the slit lamp produces inverted images of the retina with a higher resolution and a wider field of view.4 In fact, the lower the dioptric power of the lens, the higher the axial resolution and the better the stereopsis.4 This, in turn, allows for easier detection of various posterior pole pathologies including cystoid macular edema, subretinal fluid secondary to choroidal neovascularization, diabetic changes and more. In addition, careful examination of the slit lamp beam as it hits the retina can differentiate between various contours of a retinal lesion.
Advantages of this diagnostic skill include a comfortable working distance between the patient and the practitioner, various magnifications that can be obtained and creating an image independent of the refractive power of the eye.4 Finally, slit lamp indirect ophthalmoscopy can also allow for a sufficient view through small pupils or pupils that do not dilate well.
However, limitations to this procedure can include patient discomfort at the slit lamp, hazy views due to media obstruction, lack of peripheral retinal views and most importantly, difficulty appreciating the depth of a retinal lesion.4 This, in turn, may limit the description of the retinal lesion, including its thickness, level of extension and point of origination.
In contrast, optical coherence tomography (OCT) is a non-invasive imaging technique. It uses low-coherence light waves to produce high-resolution, cross-sectional images of the retina, retinal nerve fiber layer and optic nerve head.5,6 Enhanced-depth imaging (EDI) is a feature available on most OCTs that allows for better visualization of structures underneath the retinal pigment epithelium (RPE) such as the choroid.6,7 OCT is increasingly being used to analyze the retinal morphology and quantify changes in various disease states. These include, but are not limited to, macular degeneration, diabetic retinopathy and central serous retinopathy.7 The impetus for its introduction into medical practice is to document and monitor subtle retinal changes over time in an efficient manner, thereby enhancing the level of care provided.
OCT is routinely used as it is time-efficient in clinical practice, which serves to benefit doctors and patients alike. Each volumetric scan takes no more than six seconds, far less than the time it would take to complete other imaging modalities such as fluorescein angiography.7 It provides many cross-sectional images, which not only allows ODs to monitor subtle retinal changes but also serves as a visual aid to the patient.
Despite these benefits, patient motion and blinking might introduce artifacts into the imaging results and ultimately skew the quality of the image produced.7 In addition, the image quality may be limited by media opacities like cataracts or blood in the vitreous cavity.6,7 OCTs also tend to be costly and have limited field of view and limited lateral resolution, making it difficult to scan peripheral retinal lesions.7 While OCT may not be able to fully substitute a traditional funduscopic examination, it is a valuable tool in diagnosing, documenting and following a variety of ocular pathologies.
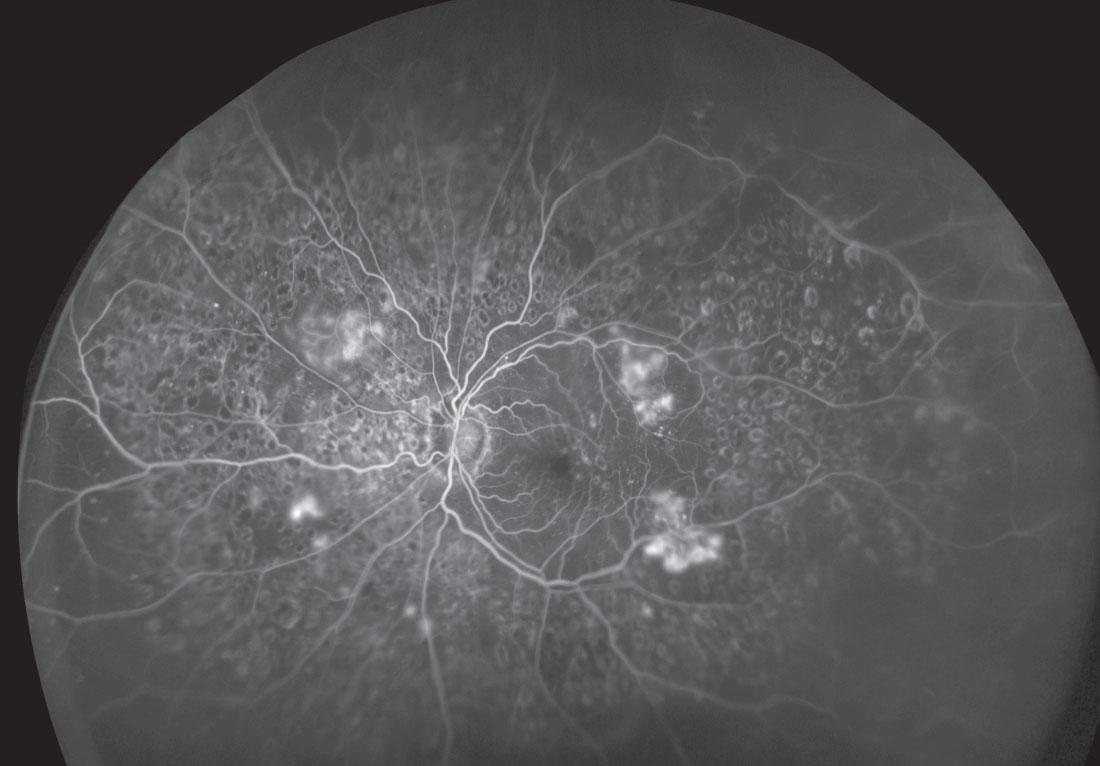 |
| This ultra-widefield image shows an expansive view of a retina with severe diabetic retinopathy. Click image to enlarge. |
Fundus Exam vs. Fundus Autofluorescence
New retinal imaging techniques can provide an in-depth analysis and a unique perspective of the retinal tissue and RPE. Specifically, fundus autofluorescence (FAF) was developed to provide functional information about the retina by imaging and assessing the underlying RPE.8 It is a non-invasive, time-efficient imaging technique that detects fluorophores, which are naturally occurring molecules that absorb and emit light of specified wavelengths.8,9 FAF employs blue-light excitation and then collects emissions within a certain spectra (typically between 500µm and 700µm) to form a brightness map reflecting the distribution of lipofuscin, a dominant fluorophore within the RPE.8 For this reason, FAF has become a useful tool to use when diagnosing and monitoring several diseases, including macular degeneration, central serous chorioretinopathy, vitelliform lesions, macular telangiectasia, medication toxicity and more.
To further understand its application, let’s look at how FAF highlights the differences between wet age-related macular degeneration (AMD) and vitelliform deposition. Choroidal neovascularization in wet AMD patients often presents with corresponding hypo-autofluorescence, which may be surrounded by a thin layer of hyper-autofluorescence reflecting a hyperplastic proliferation of RPE cells surrounding the lesion.9 Hemorrhages and exudation are initially hypo-autofluorescent due to light absorption but may eventually become hyper-autofluorescent.9 Vitelliform deposition, on the other hand, reflects an accumulation of lipofuscin underlying the macula, which creates a distinct area of hyper-autofluorescence.9,10 Subsequent RPE atrophy following vitelliform deposition corresponds to areas of hypo-autoflourescence.10
One of the most prominent advantages of this imaging modality is that it may allow for the identification of retinal diseases when they are not otherwise evident during dilated examinations. This is particularly helpful when managing patients with unknown vision loss or a positive family history of hereditary retinal diseases. For instance, early AMD encompasses alterations at the level of the RPE, which can become readily apparent in FAF imaging while appearing normal on funduscopy.9
FAF is also useful in tracking pathophysiological mechanisms. For instance, FAF can highlight areas of arterial occlusion as they inhibit proper autofluorescence of the RPE due to the increased thickness.10 Knowing this can allow for an objective analysis of a patient’s response to therapy and help monitor the natural reduction in the severity of the disease.
In addition, FAF allows for a better measurement of the progression or regression of retinal disorders compared with earlier standards of measurement. In fact, for a more precise measurement, autofluorescence can be averaged between different retinal zones or even acquired at specific desired points.10 Additionally, FAF is valuable in predicting the progression of geographic atrophy in AMD patients.9 Research shows an increased autofluorescence surrounding an area of geographic atrophy precedes its extension, a feature that cannot be appreciated during a traditional dilated examination.9
FAF, however, does have its limitations. At present, only a limited number of reference databases exist to consistently classify normal and pathological FAF phenotypes.8,9 The inter-individual and intra-individual variability of media opacities, refractive error, genetic expression and cellular lipofuscin content make the possibility to develop this database challenging.8 Differences in image acquisition not only limit producing objective autofluorescent measurements but also make the ability to compare images from different patients and operators difficult.8 Nevertheless, fundus autofluorescence is quickly being integrated into clinical practice as a complementary tool in diagnosing, following and treating retinal disorders.
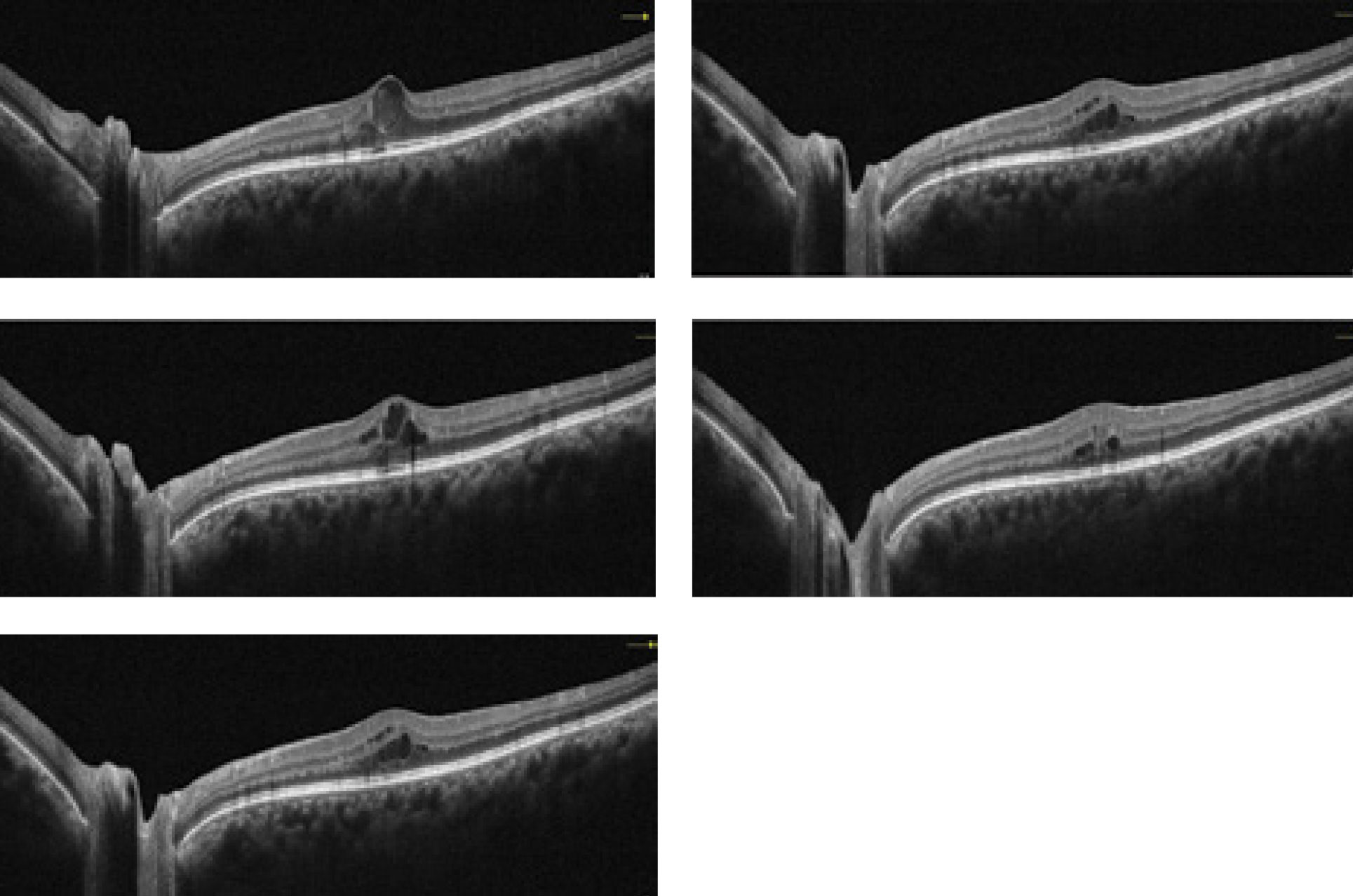 |
| This collection of OCT images exemplify how the technology is able to portray a diabetic retinopathy patient through the course of several visits. Click image to enlarge. Photos: Julie Rodman, OD. |
OCT Angiography vs. ICG Angiography
Indocyanine green (ICG) angiography is a diagnostic procedure that employs a water-soluble dye to examine the blood flow of the choroid.11 Similar to fluorescein angiography, ICG dye is injected into the vein of the arm and photographs are taken as the dye passes through the choroidal vasculature.11 Infrared light, given off by the ICG dye, has a longer operating wavelength compared with the light emitted from fluorescein dye, thereby allowing it to be better imaged through pigment, fluid, lipids and hemorrhages.11,13 This increases its ability to detect abnormalities at the level of the choroid that may otherwise be blocked by an overlying hemorrhage or hyperplastic RPE.
Additionally, ICG dye does not readily leak out of choroidal vessels, whereas fluorescein dye does.13 This gives it an advantage over intravenous fluorescein angiography (IVFA) for many choroidal pathologies. ICG angiography can also highlight abnormal aneurysmal outpouchings of the inner choroidal vascular network seen in idiopathic polypoidal choroidal vasculopathy.12
Indications for the use of ICG angiography include, but are not limited to: choroidal neovascularization, pigment epithelial detachment, polypoidal choroidal vasculopathy, central serous retinopathy, intraocular tumors and occult retinal disease.12 As an ancillary test, this dye is highly protein bound, which equates to less leakage through the choriocapillaris, reducing allergy concerns.12,13 In addition, dynamic ICG angiography uses confocal scanning laser ophthalmoscopy (SLO) to detect smaller feeder vessels, which can be valuable in establishing the role of ICG-guided therapy in medical practice.14
ICG angiography, however, does have disadvantages as well. In addition to the allergy concerns, it is invasive, expensive, time-consuming and not always readily available.12 It also has little to no benefit in other retinal vascular disorders such as diabetic retinopathy and retinal vein occlusion.13
Nevertheless, clinical applications of ICG angiography continue to expand as it allows for the analysis of choroidal blood flow in both normal and diseased eyes.14 Ultimately, this not only allows physicians to enhance current treatment strategies but also establish new management protocol for various disease states.
The retinal and choroidal vasculature can be a breeding ground for many ocular pathologies and dye-based angiography has been the gold standard diagnostic test for assessing these disorders. However, they have their limitations. OCT angiography (OCT-A), in contrast, is a novel technique that may be able to overcome some of these limitations. OCT-A is a non-invasive imaging technique that allows for the detection and three-dimensional reconstruction of the retinal capillary network.16 OCT-A images are produced by comparing, on a pixel-to-pixel basis, repeated B-scans acquired at a particular retinal location in rapid succession.16
This imaging modality has the ability to analyze each vascular capillary network individually, from the superficial to the deep plexus, which becomes crucial when studying various retinal vascular disorders such as macular telangiectasia, retinal artery and vein occlusions.15
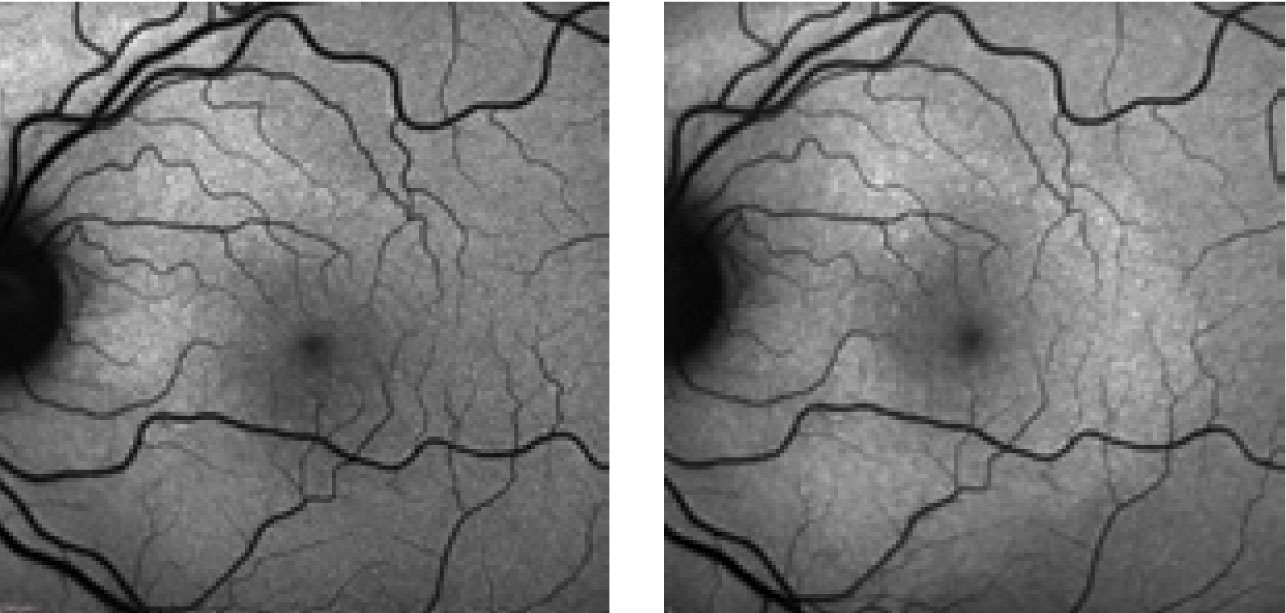 |
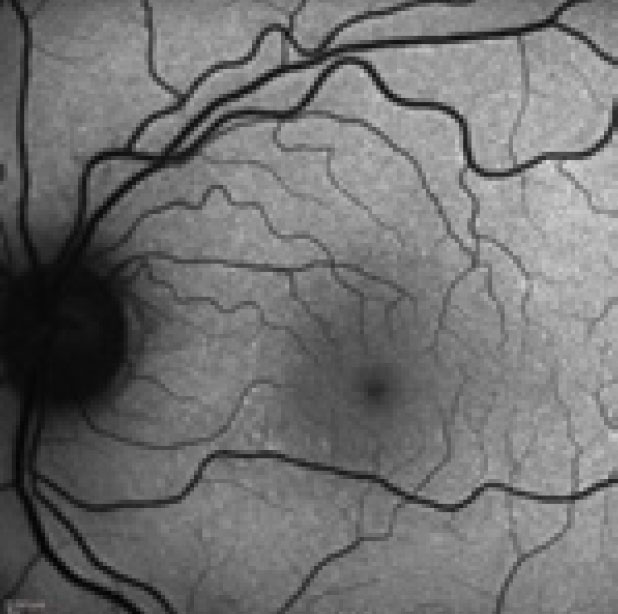 |
| These FAF photos show a patient with the changes in the photoreceptor integrity line secondary to neurosyphilis. The top left shows the patient at initial presentation; top right shows them at first follow-up; at left is their second follow-up. Note the extension of the hyperfluorescence inferior to the optic nerve and macula. Photos: Kathryn Dailey, OD. |
Additionally, OCT-A has a superior ability to precisely delineate vessels surrounding the foveal avascular zone.15,16 Being able to visualize this has provided insight into the mechanisms of various disorders. For instance, a recent study demonstrated that the perifoveal intercapillary area on OCT-A increases in size as the level of diabetic retinopathy progresses.15,17
Another important application of OCT-A is for patients with macular telangiectasia, as it is able to highlight the abnormal vessel growth in both the superficial and deep plexus, which may otherwise be unnoticed on fundus examination.15 Possibly the most important and common application is identifying different choroidal neovascularization morphologies and its response to anti-VEGF therapy. Overall, its sensitivity in cross-sectional and en face imaging make it a crucial tool in monitoring various ocular pathologies.
In its current configuration, however, there are a few limitations of OCT angiography. One drawback is that it has a restricted field of view.15 The scan protocols currently available are 2x2mm, 3x3mm, 6x6mm and 8x8mm, rendering it ineffective for peripheral retinal disorders.15,17 OCT-A is also prone to acquiring various artifacts. It works by detecting erythrocyte motion, so any extraneous motion by the patient during image acquisition can result in motion artifacts.16 In addition, low amounts of blood flow below the device threshold may go undetected.16 Media opacities can lead to signal attenuation and shadowing, thereby producing poor quality images.16,17 Superficial blood vessels may obscure abnormalities in the deeper retinal network, possibly leading to incorrect diagnoses if not promptly identified.15,16 Aside from being extremely motion sensitive, OCT-A imaging is expensive to perform.
While it is a non-invasive technique that can provide insight into the diagnosis of a retinal condition, its role in therapeutic monitoring remains unclear. Nevertheless, OCT-A is promising as it allows for the simultaneous assessment of both structural integrity and vascular flow. It is also safer, faster and more comfortable for the patient compared with dye-based approaches, making it invaluable in both clinical practice and research studies.
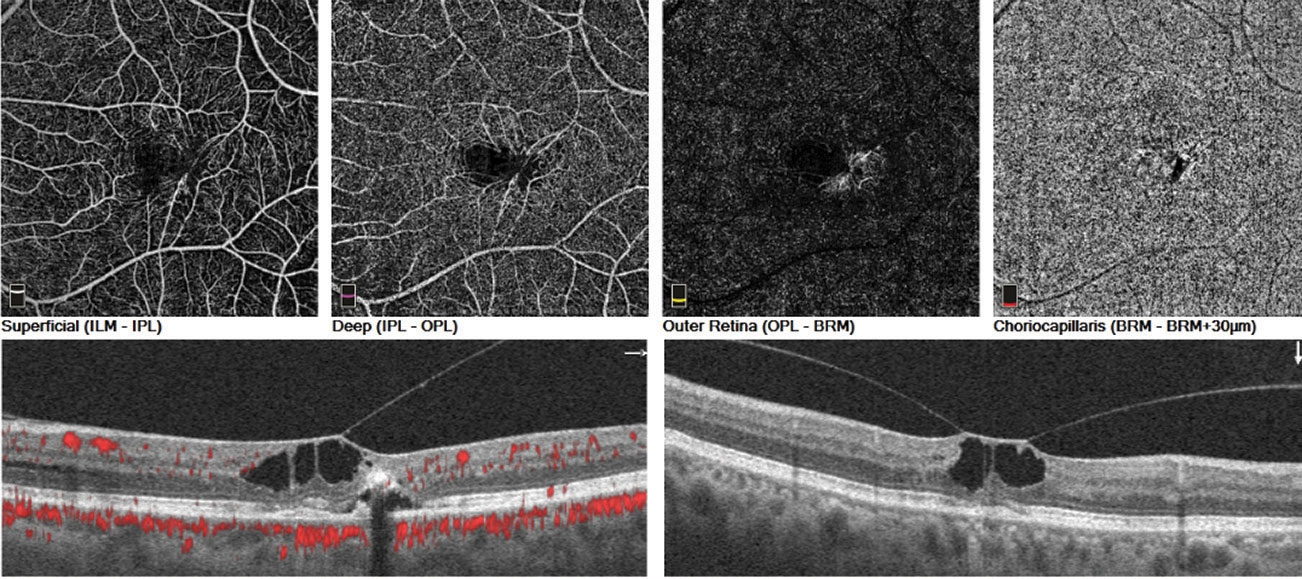 |
| These images showcase OCT angiography’s ability to image macular telangiectasia. Click image to enlarge. Photos: Julie Rodman, OD. |
A Delicate Balance
Dilation continues to be the standard of care in comprehensive optometric examinations. However, with so many new technologies on the market, optometrists can enhance their level of care and grow the profession itself. Keeping the traditional in balance with the cutting edge is key to optimizing patient care. Overall, while each piece of equipment gains traction and popularity, it is an optometrist’s duty to know how and when to employ them.
Dr. Jayasimha has completed an ocular disease residency with the Bascom Palmer Eye Institute in Miami.
1. Ellish N, Royak-Schaler R, Passmore S. Knowledge, attitudes, and beliefs about dilated eye examinations among African-Americans. Clin Ophthalmol Vis Sci. 2007;48(5):1989-94. 2. Lasave A, Shoughy S, Kozak I, Arevalo J. When ultra-widefield retinal imaging is most useful. Retina Today. 2019;14(3):36-40. 3. Walling P, Pole J, Karpecki P, et al. Condensing lenses: sharpen your skills in choosing and using. Rev Optom. 2017;154(3):58-65. 4. Cordero I. Understanding and caring for an indirect ophthalmoscope. Community Eye Health. 2016;29(95):57. 5. Fujimoto J, Pitris C, Boppart S, Brezinski. Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2(1-2):9–25. 6. Strong J. Retinal OCT imaging. www.opsweb.org/page/RetinalOCT. Ophthalmic Photographers Society. December 3, 2018. Accessed August 20,2019. 7. Jeewandara T. Optical coherence tomography (OCT)–longer wavelengths can improve imaging depths. phys.org/news/2019-02-optical-coherence-tomography-oct-longer.html. February 04, 2019. Accessed August 20, 2019. 8. Stuart A. The nuts and bolts of fundus autofluorescence imaging. American Academy of Ophthalmology. www.aao.org/eyenet/article/nuts-bolts-of-fundus-autofluorescence-imaging. September 2012. Accessed August 20, 2019. 9. Wu L. Use of fundus autofluorescence in AMD. Retina Today. 2012; 7(9): 47-52. 10. Yung M, Klufas M, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retina Vitreous. journalretinavitreous.biomedcentral.com/articles/10.1186/s40942-016-0035-x. April 8, 2016. Accessed August 20, 2019. 11. Rush R, Rush S. Current concepts of ICG angiography for evaluation of CNV. Ret Phys. 2016; 13(9):62-66. 12. Fernandez M, Gil M, Gonzalez F, Gomez-Ulla F. Diagnostic usefulness of indocyanine green angiography (ICG-A) in age-related macular degeneration (AMD). www.amdbook.org/content/diagnostic-usefulness-indocyanine-green-angiography-icga-age-related-macular-degeneration-am. July 2017. Accessed August 20, 2019. 13. Owens S. Indocyanine green angiography. Bri J Ophthamol.1996;80(3) 263-6. 14. Sarraf D. Laser video ICG angiography and SD-OCT for the diagnosis of PCV and AMD. Retina Today. 2013;8(6):83-5. 15. Gao S, Jia Y, Zhang M, et al. Optical coherence tomography angiography. Inves Ophthalmol Vis Sci. 2016;57(9):27-36. 16. Novais E, Baumal C. The clinical utility of OCT angiography. Rev Ophthalmol. 2017;24(1):51-5. 17. De Carlo T, Romano A, Waheed N, Duker J. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015;15(1):5. |

