 |
Retinal vein occlusion (RVO) is the second most common retinal vascular disease, exceeded only by diabetic retinopathy.1 RVOs can be divided into two main subtypes: branch retinal vein occlusions (BRVO) and central retinal vein occlusions (CRVO).1,2 These entities affect about 1% to 2% of all patients older than 40 and 16 million people worldwide, with BRVOs approximately five times more common than CRVOs.1-3
Despite the common presentation of RVOs, the pathogenesis is not well understood. Anatomically, the central retinal artery and vein are juxtaposed in the center of the optic nerve, enveloped in a common fibrous tissue. When this common tissue and the central retinal artery thickens and becomes sclerotic, the underlying vein is compressed. The resultant narrowing of the venous lumen creates the hemodynamic changes that lead to CRVO. In BRVO, degenerative blood vessel alterations and hemodynamic changes are caused by arteriovenous crossings and venous compression.4
Although the mechanism governing these occlusions is complicated and multifactorial, clinicians can better understand RVOs with Virchow’s triad: stagnation of blood in the blood stream, endothelial cell injury and hemodynamic changes or blood hypercoagulability.5
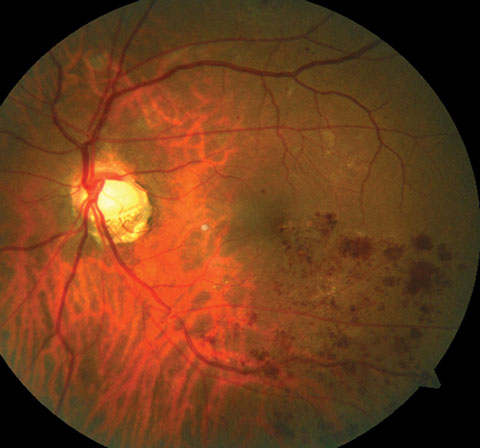 |
| This photo ilustrates a BRVO along the inferior temporal arcade. |
Historical Perspective
In 1856, Rudolf Virchow first introduced the theory that a triad of physiological factors plays a significant role in the etiology of venous thrombosis.6-8 Today, researchers still believe blood stagnation, endothelial cell injury and hypercoagulability—occurring separately or concurrently—increase a patient’s risk for venous thrombosis.6 The most recognized risk factors for RVO, besides advancing age, are systemic vascular disorders.2 Several studies report systemic causes of hypercoagulability, as well as diseases that increase risk of endothelial damage are associated with RVOs.1,4,5,9 Here is a closer look at the triad and the risk factors that may predispose a patient to developing RVO.
1. Hemostasis/abnormal blood flow. A 1976 study originally described the milder forms of CRVO as “venous stasis retinopathy,” differing from the condition we often refer to today as the midperipheral retinal hemorrhages associated with early ocular ischemia secondary to carotid artery stenosis.10-12 This sets the stage for the first component of Virchow’s triad, stagnant blood flow. As blood viscosity is often raised at the time of occlusion, anything that contributes to blood viscosity may play a significant role in RVO. Research shows that hemostasis increases blood viscosity and platelet aggregation, highlighting its role in the pathogenesis of RVO.5,9,10 Rarely, myeloproliferative disorders (cancers), pregnancy, use of oral contraceptives and systemic vasculitides that also result in blood hyperviscosity have been known to cause RVO, especially in younger individuals.13
Virchow’s Triad | Systemic Associations |
| 1. Hemostasis |
|
| 2. Hypercoagulability |
|
| 3. Damage to blood vessel wall | Arteriosclerosis secondary to:
|
| Here, the components of Virchow’s triad are associated with systemic disorders that carry risks for the development of retinal vein occlusions. | |
2. Hypercoagulability. Research shows systemic causes of hypercoagulability significantly increase risk of CRVO.3,9 These include: protein C and S deficiency, antithrombin III, factor V Leiden, hyperhomocysteinemia, thrombophilia and anticardiolipin antibodies.3,9 However, these conditions are not as commonly linked to BRVO formation, which are often a result of the adjacent, compressive arteriolar changes.9
3. Degenerative changes to blood vessel wall. Researchers have extensively studied the histological changes in retinal blood vessel walls, especially at the site of arteriovenous crossing, and found hypertrophy of the intima media in 90% of BRVO cases.5 These trophic changes are the initiation of RVOs, ultimately leading to thrombus formation. Hypertension, hyperlipidemia and diabetes mellitus are the main systemic risk factors of RVO, as patients with hypertension alone have an increased risk for BRVO—78% according to one study.9 This is because these conditions cause or contribute to arteriosclerosis, resulting in the compression of adjacent retinal veins and venous stasis.9
In The Clinic
RVOs may cause profound visual deficits that require prompt recognition and treatment. However, it is just as, if not more, important to identify the various systemic risk factors and pathogenesis of thrombus formation to prevent serious consequences, including mortality. Understanding the several causes of RVOs, which is best summarized as Virchow’s triad, will allow the clinician to develop a systematic approach for screening for the underlying pathogenesis. For patients with known metabolic syndrome or one of those components—diabetes mellitus, hypertension and hyperlipidemia—clinicians must stress the importance of tight control and treatment as preventative methods.9 If metabolic syndrome is absent following initial laboratory work up (e.g., lipid panel, hemoglobin A1c and blood sugar testing), for example in a younger individual, evidence suggests further screening for hypercoagulable states and thrombophilia is warranted through additional blood tests (e.g., complete blood count).13
The multifactorial nature of RVO pathogenesis highlights the significance of a holistic approach.
Although the underpinnings of RVO are poorly understood, a historically grounded concept of the disease state can help clinicians diagnosis occlusion in the eye and, systemically, prevent serious risk associated with thrombus.
|
1. Kolar P. Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J Ophthalmol. 2014;2014(6):1-5. 2. Kolar P. Definition and classification of retinal vein occlusion. Inter J Ophthal Res. 2016(6):1-12. 3. Wong TY, Scott IU. Retinal-vein occlusion. NEJM. 2010;363:2135-44. 4. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33(2):111-31. 5. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24(4):493-519. 6. Kumar DR, Hanlin E, Glurich I, et al. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clinical Medicine and Research. 2009;8(3):168-72. 7. Zoppo GJ. Virchow’s triad: the vascular basis of cerebral injury. Rev Neurol Dis. 2008;5(supp 1):S12-21. 8. Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. 2009;23(5):225-9. 9. Newman-Casey PA, Stem M, Talwar N, et al. Risk factors associated with developing branch retinal vein occlusion among enrollees in a United States managed care plan. Ophthalmology. 2014;121(10):1939-48. 10. Williamson TH. Central retinal vein occlusion: what’s the story? Br J Ophthalmol. 1997;81(8):698-704. 11. Hayreh SS. So-called ‘central retinal vein occlusion’. II. Venous stasis retinopathy. Ophthalmologica 1976;172(1):14-37. 12. Guyennett E, et al. Venous stasis retinopathy and carotid artery stenosis. Invest Ophthalmol Vis Sci. 2008;49(18):1-2. 13. Karia N. Retinal vein occlusion: pathophysiology and treatment options. Clin Ophthalmol. 2010;4:809-816. |

