Innovations in Eye CareCheck out the other feature articles in this month's issue:A Light in the DARC: Seeing Glaucoma Before it Strikes Iontophoresis: Wave of the Future Can We Lower IOP with Glasses? While You Were Sleeping |
Over the last several decades, advances in stem cell biology have opened the door to cutting-edge clinical trials aimed at treating age-related macular degeneration (AMD), one of the leading causes of blindness in the western world.1 Research focuses on two main categories of stem cells, human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSC). hESC are derived from the early blastocyst stage of a fertilized human egg that has been donated to research. They have the ability to differentiate indefinitely and can become any cell type in the body. In contrast, iPSC are derived from adult human cells, usually skin cells. These cells are reprogrammed in the lab to revert to the pluripotent state from which they can be differentiated into almost any cell type.
While there are many differences between these two types of cells, one major distinction is that iPSC can be obtained from the same person who needs the stem cell-based therapy, thereby minimizing the chances of an immune response. However, creating iPSC can be a labor-intensive and expensive process that is not currently commercially feasible. Nevertheless, both cell types have been used to generate replacement tissue for subjects with atrophic retinal pigment epithelium (RPE) and AMD. Here we discuss the current research efforts to harness the healing powers of stem cell therapy for future AMD treatment.
Treatment Needs
AMD is classified as either neovascular (NVAMD) or non-neovascular (NNAMD), also called wet and dry, based on the presence or absence of neovascularization, respectively.2 Patients with wet AMD have several treatment options, including intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs, laser photocoagulation to ablate the neovascular membrane, surgical removal of the neovascular membrane with or without retinal translocation and photodynamic therapy.3 Applications of stem cell-based therapy in NVAMD are likely limited to cases involving RPE tears or RPE damage from recurrent subretinal hemorrhage.
Unlike its neovascular counterpart, dry AMD lacks therapeutic options and causes debilitating vision loss, albeit over decades. Progression and vision loss is characterized by focal loss of the RPE and atrophy of the associated overlying neurosensory layer, a process also known as geographic atrophy (GA).4 In theory, being able to regenerate and replace the damaged RPE through stem cell-based treatments could delay or even reverse the consequences of GA.
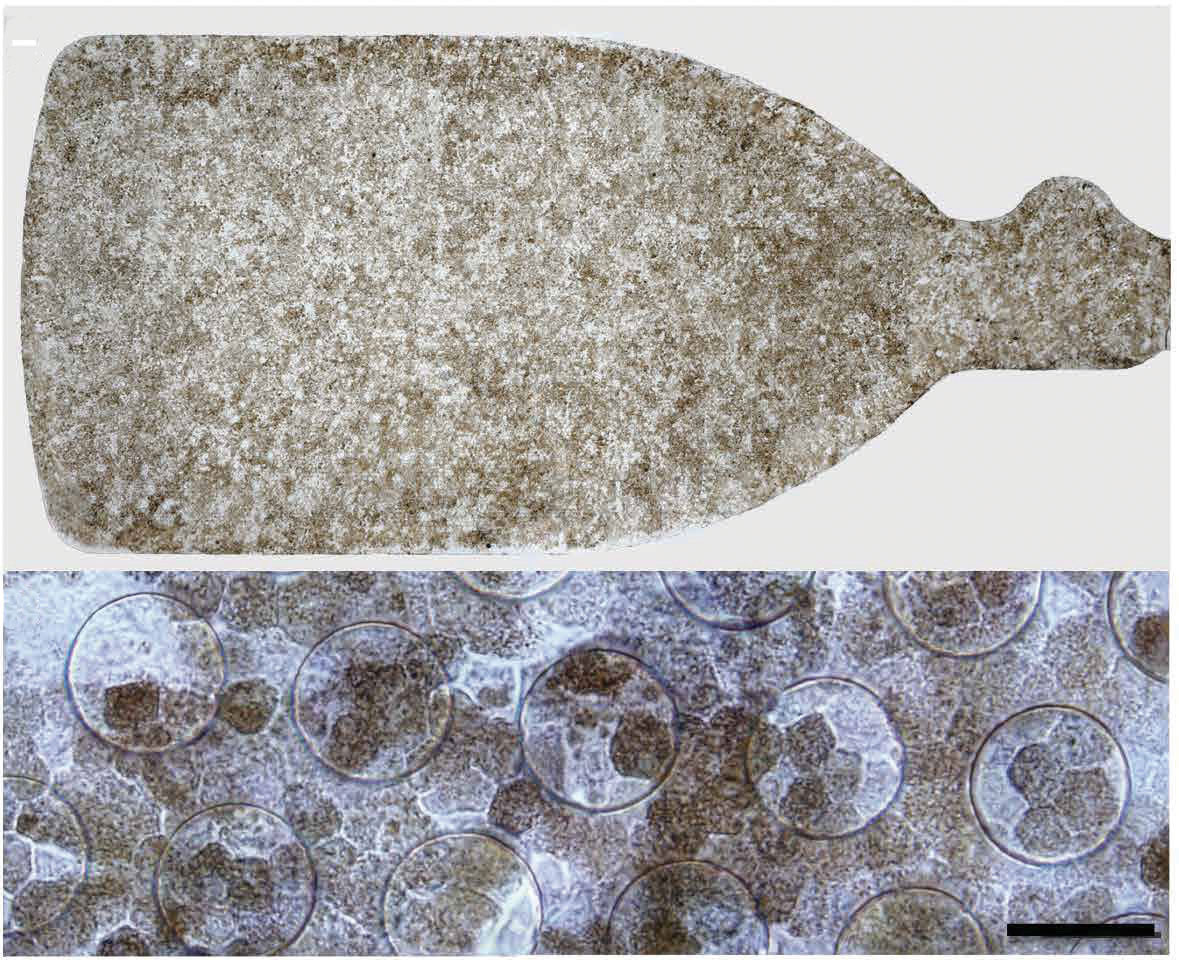 |
| Fig. 1. Above is a 3.5mm × 6.25mm sheet of hESC-RPE. Below is the same implant under high-magnification.6 Reprinted from Kashani AH, Lebkowski JS, Rahhal FM, et al |
Research Efforts
Over the past year, three early-phase trials have investigated the effects of replacing damaged RPE in subjects with NVAMD and NNAMD with stem cell-derived counterparts—each with exciting results.
The earliest of the three studies, reported in the New England Journal of Medicine in 2017, included one patient with NVAMD who had severe vision loss and was unresponsive to conventional therapy.3 As part of the trial, the patient received a transplant consisting of iPSC-RPE. A 1.3mm x 3.0mm sheet of iPSC-RPE created using the patient’s skin fibroblasts was surgically placed under the fovea. Unfortunately, the researchers observed no change in visual acuity during the postoperative one-year follow-up, and retinal sensitivity also remained unchanged. However, a modest improvement on the National Eye Institute Visual Function Questionnaire 25 (VFQ-25) and a more foveal-centric fixation point showed some promise.
Further study with a second patient was hampered by several complications, beginning with the concern for potential medical risk due to genetic alterations. In addition, the cost of the iPSC-RPE differentiation procedure was prohibitive for additional studies, and despite the sophisticated surgical methods used in the study, the iPSC-RPE sheet was difficult to handle intraoperatively.
Further analysis revealed one more complication, this time with the biocompatibility of the sheet itself. Imaging of the postoperative anatomy of the sheet showed it did not automatically conform to the anatomy of the native RPE monolayer. Despite these setbacks, the researchers felt this heroic attempt at replacing damaged RPE demonstrated the successful creation and surgical implantation of iPSC-RPE. The results provided enough information to keep investigations moving forward, as there was no evidence of transplant rejection or ill effects of the implant on the subject.
In April 2018, another study, reported in Nature Biotechnology, included two patients with wet AMD and severe vision loss who were treated with hESC-RPE cells.5 In this study, the surgeon used an artificial polymer to support the RPE monolayer, which helped with the surgical delivery of the cells into the area where the RPE had been damaged or ripped as a result of the NVAMD process. Of the 10 patients enrolled in this study, two received the transplantation and were included in published findings to date. At the one-year follow-up, spectral-domain optical coherence tomography (SD-OCT) demonstrated proper orientation and persistence of the RPE layer relative to the surrounding tissue. The researchers also noted evidence of significant RPE migration away from the implantation site, for unclear reasons; but importantly, they saw no indication of transplant rejection. At least one subject required further surgery to repair a retinal detachment from proliferative vitreoretinopathy following implantation—a complication that illustrates the difficulty of the surgical procedure.
Most recently, our group reported preliminary results in Science Translational Medicine on five patients with severe vision loss from advanced dry AMD and GA.6 These patients were implanted with a ~3mm x 6mm sheet of hESC-RPE on a biosynthetic substrate that mimics the native Bruch’s membrane in several aspects (Figure 1). Most importantly, this substrate has permeability properties that allow nutrients and oxygen to diffuse back and forth between the retina, RPE and choriocapillaris. The interim results of this phase 1/2a study demonstrated that the procedure was well-tolerated and three of the five subjects showed signs of visual function improvement. While the small sample size hinders our ability to determine whether the improvement is statistically or clinically significant, one patient maintained a 17-letter improvement, as well as improved fixation, over three follow-up visits.
Postoperative OCT imaging demonstrated a monolayer of RPE that is quite similar to the native anatomy of the hosts’ RPE, suggesting the implant is integrating with or supporting the overlying host retina where viable photoreceptor inner and outer segments are present at the edge of the GA (Figure 2).7 This preliminary data suggests short-term safety of the implant, as well as its potential efficacy.
The study is still ongoing, with 16 subjects currently enrolled. Similar to the two previous studies, we have noted no evidence of transplant rejection or ill effects on the subjects, although we have yet to report on the final, long-term results.
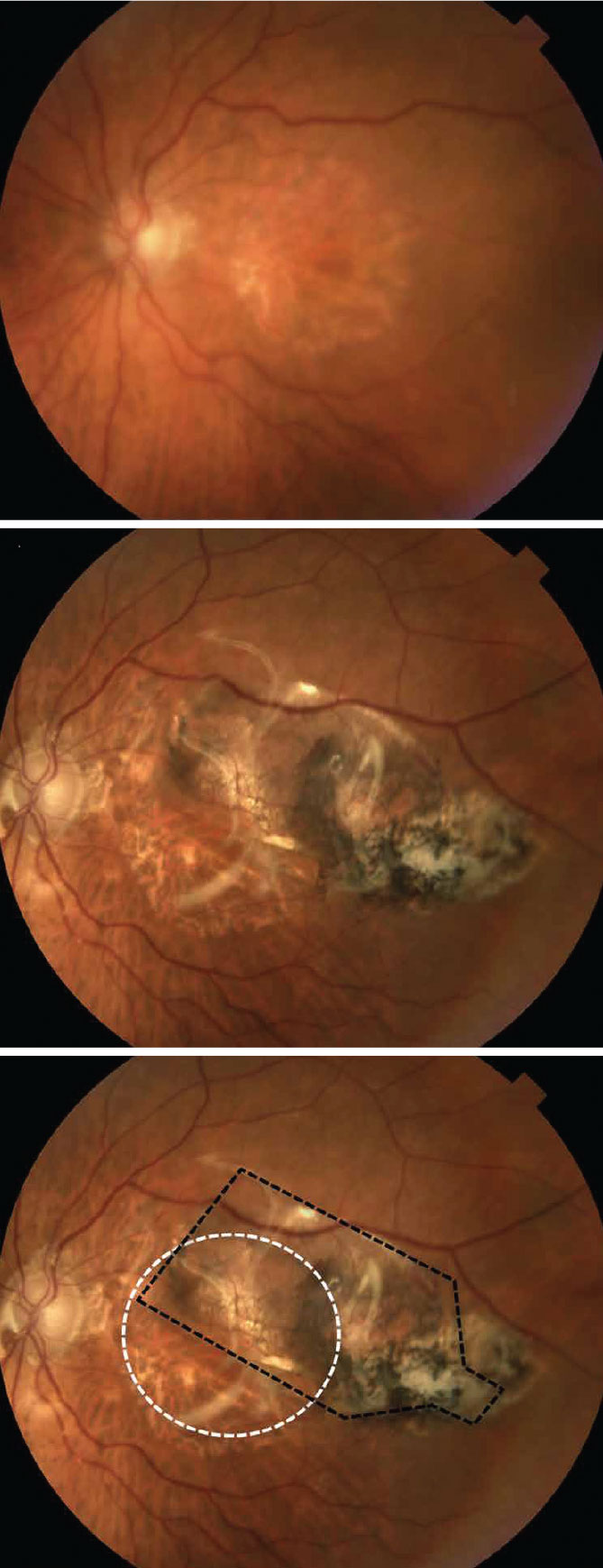 |
| Fig. 2. Above, the study subject’s pre-op fundus photo shows large areas of RPE loss, consistent with GA. Middle, the fundus photo 180 days after implantation with a sheet of hESC-RPE.6 Bottom, the annotated post-op image shows the location of GA (white dashed line) and the implant (black dashed lines). Reprinted from Kashani AH, Lebkowski JS, Rahhal FM, et al |
Surgical Concerns
The surgical methods involved in these studies can be challenging, and proper patient selection is critical to their success.4 Subjects with GA have generally had long-standing disease and atrophy of the photoreceptor layer in addition to the RPE. This often causes the remaining retina to be highly adherent to the underlying aberrant Bruch’s membrane, making surgical separation of the retina formidable. Careful surgical planning, patient selection and innovative surgical tools are needed to avoid potentially serious complications such as proliferative vitreoretinopathy, which has confounded previous submacular surgeries as is described in the submacular surgery trials for NVAMD.5
With the advent of modern, small-gauge vitreoretinal surgery and equipment, the success rates have increased, while complication rates for surgery have dramatically decreased over the past few decades. In all of the studies reported here, custom surgical instruments were designed and implemented for delivery of the RPE implants. It is likely that the current challenges facing stem cell-derived RPE implantation will be overcome with the aid of technological advances.
The results of these exciting studies lay the groundwork for a promising future in AMD treatment using stem cell-derived therapies. The lack of serious and unanticipated adverse events suggests that the cell-based therapies, when done correctly, are well-tolerated and a feasible therapeutic modality.
The efficacy of these treatments remains to be proven, and several research hurdles remain. Therefore, follow-up studies will look closely at the data to determine the correct patient selection criteria, as well as what clinical criteria may define efficacy. For example, because GA patients often have chronic disease and overlying retinal atrophy, improvements in traditional visual acuity may be limited in these end-stage cases. Despite this limitation, three subjects in our study still showed signs of improvement. The favorable outcomes suggest these subretinal procedures may be warranted, even in later stages of the disease. Therefore, achievable clinical end-points other than acuity must be identified to guarantee a benefit to potential prospective patients.
Overall, the future of stem cell-based retinal therapy is bright, and a great deal of hope exists for patients suffering from what is currently an untreatable and blinding disease.
Dr. Kashani is an assistant professor at the University of Southern California’s (USC) Roski Eye Institute and Keck School of Medicine of USC. He is a member of the Institute for Biomedical Therapeutics.
Ms. Hong, a medical student at the California Northstate University, is interested in purusing ophthalmology and is doing a research year elective at the USC Roski Eye Institute.
Dr. Humayun is a professor of ophthalmology and biomedical engineering at the USC Roski Eye Institute and Keck School of Medicine of USC. He is co-director of the USC Roski Eye Institute and director of the Institute for Biomedical Therapeutics.
Financial disclosure: The sponsor, Regenerative Patch Technologies (RPT), and the California Institute for Regenerative Medicine provided grant support to USC. Dr. Humayun has intellectual property related to this study.
1. Rein DB, John S. Wittenborn JS, Zhang X; Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050—the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533-40. 2. Nazari H, Zhang L, Zhu D, et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Progress in Retinal and Eye Research. 2015;48:1-39. 4. Kashani AH. Stem cell therapy in nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFm1-9. 3. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038-46. 5. da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36(4):328-37. 6. Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Science Translational Medicine. 2018;10(435). 7. Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Progress in Retinal and Eye Research. 2017;60:66-100. |

