In recent decades, diabetic retinopathy (DR) and myopia have been increasing in prevalence; both contribute greatly to visual impairment.1-4 Diabetes has been linked to changes in refractive error under hyperglycemic conditions. It is essential to understand this relationship when determining the proper refractive correction; hyperopic rather than myopic shift is the more common effect, although the literature has been somewhat contradictory on that in the past.5-19 Interestingly, refractive error—especially high myopia—may actually decrease the progression of DR, even though it is associated with serious ocular complications, such as an increased risk of retinal detachment.20-24
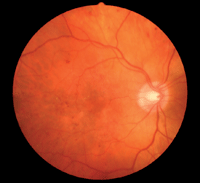
Diabetic retinopathy in a moderately myopic patient (-5.00D). Research has shown an inverse relationship between myopia level and risk of proliferative DR.
This paper will summarize the relationship between refractive status and fluctuations in glucose levels, and attempt to explain the different theories that various authors have proposed over the years. This review also will help us learn more details about the relationship between refractive error and DR pathogenesis, to help us uncover the mechanisms of a possible protective effect in highly myopic eyes.
Hyperglycemia and Refractive Error
The poor metabolic control of blood glucose that diabetes patients experience, particularly hyperglycemia, leads to changes in the eye’s refractive status. Although the effect on refraction has been investigated by many studies—some dating back at least to 1925—there is no agreement in the literature on the mechanism or the typical disease course. Indeed, both hyperopic and myopic shifts in subjects with diabetes have been documented by several studies.
Notable additions to the literature on myopic shift under hyperglycemic conditions have come from Duke-Elder (1925), Birnbaum & Leu (1975), Gwinup & Villarreal (1976), Fledelius (1990) and Furushima (1999). Scholars documenting hyperopic shift during intensive treatment of acute metabolic dysregulation include Planten (1975 & 1978), Kluxen & Scholz (1987), Saito (1993), Okamoto (2000), Giusti (2003) Sonmez (2005) and Tai (2006).5-17 Verma (1980) and Herse (2005) induced hyperglycemia in rabbits and found hyperopic shift upon refraction.18,19
Duke-Elder suggested that hyperglycemia causes myopic shift, while a decrease in blood glucose levels leads to hyperopic shift due to the osmotic force between the crystalline lens and the aqueous humor that results from changes in molecular concentration.5 This same theory was suggested by Gwinup (1976), who studied the phenomenon in 10 subjects with diabetes, including four aphakic eyes, and concluded that higher and lower glucose levels produce myopia and hyperopia, respectively.7 Although a few early studies had suggested that hyperglycemia leads to myopic shift, noting that the eye’s refractive error shifted towards hyperopia as the glucose level reduced, later studies observed precisely the opposite—during hyperglycemic states, the refractive shift is more likely hyperopic. Fledelius (1990) studied 15 subjects with high glucose levels and documented refractive fluctuation of 1.00D to 6.50D toward hyperopia.8
Some studies in which hyperglycemia was induced have shown a change in the thickness and/or curvature of the lens, altering its refractive index. Sito and associates (1993) stated that thickening of the lens correlates with the refractive shift towards hyperopia. This team also found a decrease in anterior chamber depth.13 Huggert (1953-54) studied ways in which diabetes affects the crystalline lens and suggested that changes occur anterior to the nucleus.22,23 Both in early-onset and late-onset diabetes, there is change in lens thickness when compared with healthy, subjects without diabetes.24,25 Kluxen and Scholz (1987) reported a case demonstrating enlargement of the axial diameter of the cortex and nucleus, as revealed by Scheimpflug photography during an episode of transient hyperopia.12
After inducing acute hyperglycemia in seven healthy subjects, Furushima (1999) found an increase in lens thickness of 1mm and a myopic shift of 2.00D.9 Kato (2000) reported a significant increase in lens thickness (0.3mm) after rapid control of hyperglycemia.26 In a small study, Wiemer (2009) reported that one of his five subjects experienced hyperopic shift during induced acute hyperglycemia, but there was no change of refractive status in the other four cases.27 The authors reiterated the theory that the hyperopic shift in their one case could have been due to changes in the shape of lens. Lending credence, their report also showed no change to either the anterior and posterior corneal curvature.
In a study investigating the optical properties of LeGrand’s “full theoretical eye,” Planten (1975) demonstrated that a decrease in the refractive index of the lens from 1.42 to 1.40 would produce a hyperopic change of 3.20D.11 The same author found changes in lens thickness, as measured by A-scan ultrasound, and suggested the hyperopic shift was caused by changes in the refractive indices of the different layers of the lens.10
Herse (2005) found a similar hyperopic shift in rabbit eyes and suggested it could be due to changes in the refractive index of the cortical fibers of the lens.19 It is important to note that blurred vision during hyperglycemia also can be caused by factors other than refractive changes.26 For instance, changes in metabolism can also lead to alterations in media transparency and autofluorescence. In addition, tear film instability secondary to increased tear osmolarity can reduce visual acuity in patients with poorly controlled diabetes.28
Both corneal and lens autofluorescence are higher in the diabetic population than in healthy control groups because of fluorophore accumulation in the cornea and lens of diabetic patients, which is increased in proportion to the level of glycemic control. The concentration of glucose in aqueous humor increased along with glucose levels when there was a breakdown of the blood-aqueous barrier, giving rise to autofluorescence.29
Although many of the studies cited above noted refractive shifts in hyperglycemic subjects, bear in mind that only a few found significant changes in refractive error, and the effect tends to be transient. The physiology of the anterior segment and chamber––and their relationship to refractive error during hyperglycemia––still need to be studied in greater detail, but the prevailing theory seems to be that hyperopia might result from intraocular alternations of the lens that change its thickness and hence its refractive index.
Refractive Error and the Myopic Retina
High myopia is associated with progressive elongation of the globe, resulting in a variety of fundus changes that lead to visual impairment, including lacquer cracks in Bruch’s membrane, choroidal neovascularization and chorioretinal atrophy. Increased axial elongation in myopes may also lead to mechanical stretching and thinning of the choroid and retinal pigment epithelium, resulting in posterior staphyloma.30,31
Histopathologic studies in postmortem eyes indicate that occlusion and disappearance of large choroidal vessels and capillaries, and consequent replacement of the normal choroidal structure with fibrous tissue, are common.32-34
Myopic refractive error and longer axial length are associated with narrower retinal arterioles and venules, less tortuous arterioles and increased branching coefficients in both arterioles and venules.35 This reduces retinal blood flow and, as a result, can potentially decrease the risk of DR development. Complete posterior vitreous detachment (CPVD) was more frequently noted in myopic eyes and this finding was associated with reduced progression of neovascularization.
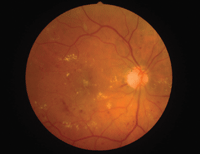
Hyperopic patients with diabetes may be at higher risk for sight-threatening retinopathy, as in this individual with a +1.00D prescription.
DR usually develops in a symmetric pattern over a long period of time; development of asymmetric DR is exceptionally rare. However, high myopia is one finding that can be responsible for asymmetric DR retinopathy, along with other ocular factors such as optic atrophy and PVD, and systemic factors such as glycosylated hemoglobin level and high blood pressure. The Early Treatment of Diabetic Retinopathy Study defined asymmetric DR as proliferative diabetic retinopathy (PDR) in one eye and non-proliferative diabetic retinopathy (NPDR) or no retinopathy in the fellow eye persisting for more than two years.36 Asymmetric DR occurs in 5% to 10% of diabetic patients with PDR.37
Some studies of anisometropic cases (defined as a difference of more than 1.00D between eyes) have shown that the eye with the higher level of myopia had a lesser degree of retinopathy, while the fellow eye typically demonstrated a higher degree of retinopathy.38,39 This showed that anisometropia plays a role in asymmetric DR presentations, and suggests that the degree of refractive error is inversely related to the severity of DR.
Dogru (1998) showed that high myopia along with optic atrophy leads to a decreased risk of PDR.40 He suggested that high myopia associated with elongation of the eyeball, and atrophy leads to a decrease in blood flow in the retinal blood vessels.
High Myopia as a Protective Factor
There are some clinic- and population-based studies showing that myopia—especially higher myopia—has a protective effect against DR.41,42 Both of the population-based studies, the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) and Singapore Malay Eye Study (SiMES), suggested that high myopia reduces the progression of DR.
WESDR defined myopia as spherical equivalent of -2.00D or more and found that myopia was associated with a lower risk of development of PDR in younger-onset diabetes. The authors suggested that myopia confers a protective factor through axial globe elongation, deformation of the posterior pole and alteration of the ocular blood flow.
SiMES defined low myopia as a spherical equivalent (SE) between -0.50D and -5.00D, high myopia as -5.00D or more SE, and hyperopia as >1.00D SE. The study’s results suggested that highly myopic patients, with a deeper anterior chamber depth and longer axial length, have a lower risk of developing DR, and particularly sight-threatening PDR.
Eyes with myopia are more likely to have a longer axial length, elevating the risk of complete vitreous detachment. Shorter rather than longer axial length is a risk factor for a higher level of DR.43 Partial posterior vitreous detachment with vitreous traction is a high risk factor for development of PDR. Incomplete PVD is usually associated with severe fibrovascular proliferation.44,45 The vitreoretinal traction causes the progression of new blood vessels and thickening of existing fibrous proliferation.45 Thus, incomplete PVD results in an elevated risk of developing PDR as well as vitreous hemorrhage, retinal detachment and retinal breaks. In complete vitreous detachment without vitreous traction, there is less chance of such progression.47
In myopia, complete posterior vitreous detachment and vitreous syneresis are more common than other refractive errors, and they protect against development of PDR by reducing the likelihood of neovascularization progression.47-50 Reports also have suggested that vitrectomy may be a protective factor against diabetic macular edema in cases of vitreomacular traction.51
Conclusion
A thorough review of the literature suggests that is it more likely that hyperopia rather than myopia occurs in cases of uncontrolled diabetes. Greater understanding of the predisposing factors and mechanisms would improve our ability to properly manage these cases.
Patients may require frequent changes of spectacles in the presence of persistently high hyperglycemia. Consequently, optometrists are advised to delay the prescription of new glasses as long as possible until stabilization of refraction (and glucose levels) is noted; potential benchmarks of stabilization to consider are an HbA1C below 7.5% and a casual in-office blood glucose of less than 180mg/dl. We must also educate our patients about the causal relationship between poor blood glucose control and the risk of severe retinopathy as well as significant vision loss.
Even though high myopia may be associated with an increased risk of ocular complications, it can act as a protective factor that forestalls proliferative DR. Lastly, be mindful to consider high myopia as a risk factor for asymmetric DR.
Dr. Bhandari is a lecturer on the optometry faculty at Twintech International University College of Technology in Kuala Lumpur, Malaysia.
1. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047-53.
2. Yach D, Alberti G: Screening for Type 2 Diabetes. 2003; World Health Organization, Geneva.
3. Grosvenor T. Why is there an epidemic of myopia? Clin Exp Optom. 2003;86:273-75.
4. Saw SM, Gazzard G, Chan SY, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:1-11.
5. Duke-Elder S. Changes in refraction in diabetes mellitus. Br J Ophthalmol 1925;9:167-87.
6. Birnbaum F, Leu P. Acute myopia with increased intraocular pressure due to a decompensated juvenile diabetes mellitus. Klin Monatsbl Augenheilkd. 1975;167:613-5.
7. Gwinup G, Villarreal A. Relationship of serum glucose concentration to changes in refraction. Diabetes. 1976;25:29-31.
8. Fledelius HC, Fuchs J, Reck A. Refraction in diabetics during metabolic dysregulation, acute or chronic. With special reference to the diabetic myopia concept. Acta Ophthalmol (Copenh). 1990;68:275-80.
9. Furushima M, Imaizumi M, Nakatsuka K. Changes in refraction caused by induction of acute hyperglycaemia in healthy volunteers. Jpn J Ophthalmol. 1999;43:398-403.
10. Planten JT: Physiologic optic approach of lens and cataract. Ophthalmologica 1975;171:249-53.
11. Planten JT, Kooijman AC, De Vries B, Woldringh JJ. Pathological-optic approach of cataract and lens. Ophthalmologica 1978;176:331-4.
12. Kluxen G, Scholz A. Evaluation of Scheimpflug photographs in transitory hypermetropia. Klin Monatsbl Augenheilkd. 1987;191:129-32.
13. Saito Y, Ohmi G, Kinoshita S, et al. Transient hyperopia with lens swelling at initial therapy in diabetes. Br J Ophthalmol. 1993;77:145-8.
14. Okamoto F, Sone H, Nonoyama T, Hommura S. Refractive changes in diabetic patients during intensive glycaemic control. Br J Ophthalmol. 2000;84:1097-102.
15. Giusti C. Transient hyperopic refractive changes in newly diagnosed juvenile diabetes. Swiss Med Wkly. 2003;133:200-5.
16. Sonmez B, Bozkurt B, Atmaca A, et al. Effect of glycaemic control on refractive changes in diabetic patients with hyperglyaemia. Cornea. 2005;24:531-7.
17. Tai MC, Lin SY, Chen JT, et al. Sweet hyperopia: refractive changes in acute hyperglycaemia. Eur J Ophthalmol. 2006;16:663-6.
18. Varma SD, El-Aguizy HK, Richards RD. Refractive change in alloxan diabetic rabbits. Control by flavonoids I. Acta Ophthalmol (Copenh). 1980;58:748-59.
19. Herse P. Effects of hyperglycemia on ocular development in rabbit: refraction and biometric changes. Ophthalmic Physiol Opt. 2005;25:97-104.
20. Browning DJ, Flynn HW, Blakenship GW. Asymmetric retinopathy in patients with diabetes mellitus. Am J Ophthalmol. 1988;105:584-9.
21. Dogru M, Inoue M, Nakamura M, Yamamoto M. Modifying factors related to asymmetric diabetic retinopathy. Eye (Lond). 1998;12(Pt 6):929-33.
22. Lim LS, Lamoureux E, Saw SM, et al. Are myopic eyes less likely to have diabetic retinopathy? Ophthalmology. 2010 Mar;117(3):524-30.
23. Moss SE, Klein R, Klein BE. Ocular factors in the incidence and progression of diabetic retinopathy. Ophthalmology 1994;101:77-83.
24. Sparrow JM, Bron AJ, Brown NA, Neil HA. Biometry of the crystalline lens in early-onset diabetes. Br J Ophthalmol. 1990;74:654-60.
25. Fledelius HC, Miyamoto K. Diabetic myopia — is it lens-induced? An oculometric study comprising ultrasound measurements. Acta Ophthalmol (Kbh). 1987;65:469-73.
26. Kato S, Oshika T, Numaga J, et al. Influence of rapid glycemic control on lens opacity in patients with diabetes mellitus. Am J Ophthalmol. 2000 Sep;130(3):354-5.
27. Wiemer NG, Dubbelman M, Ringens PJ, Polak BC. Measuring the refractive properties of the diabetic eye during blurred vision and hyperglycemia using aberrometry and Scheimpflug imaging. Acta Ophthalmol. 2009 Mar;87(2):176-82.
28. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 2001;108:586-92.
29. Bhandari M, Raman R, Sharma T. Clinical application of ocular fluorophotometer. Exp Rev Opthalmol. 2011;6(2):159-63.
30. Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:3876-80.
31. Pierro L, Camesasca FI, Mischi M, Brancato R. Peripheral retinal changes and axial myopia. Retina. 1992;12(1):12-7.
32. Ohno H. Electron microscopic studies of myopic retinochoroidal atrophies 1. Choroidal changes (in Japanese). Fol Ophthalmol Jpn. 1983;43:1244-53.
33. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina 1992;12:127-33.
34. Okabe S, Matsuo N, Okamoto S, Kataoka H. Electron microscopic studies on retinochoroidal atrophy in the human eye. Acta Med Okayama 1982;36:11-21.
35. Lim LS, Cheung CY, Lin X, et al. Influence of refractive error and axial length on retinal vessel geometric characteristics. Invest Ophthalmol Vis Sci. 2011 Feb 3;52(2):669-78.
36. Early Treatment of Diabetic Retinopathy Study Group. Ophthalmology 1991;98:739-834.
37. Valone JA Jr, McMeel JW, Franks EP. Unilateral proliferative diabetic retinopathy. I. Initial Findings. Arch Ophthalmol. 1981;99:1362-6.
38. Browning DJ, Flynn HW, Blakenship GW. Asymmetric retinopathy in patients with diabetes mellitus. Am J Ophthalmol. 1988;105:584-9.
39. Valone JA Jr, McMeel JW, Franks EP. Unilateral proliferative diabetic retinopathy. II. Clinical course. Arch Ophthalmol. 1981 Aug;99(8):1362-6.
40. Dogru M, Inoue M, Nakamura M, Yamamoto M. Modifying factors related to asymmetric diabetic retinopathy. Eye (Lond). 1998;12(Pt 6):929-33.
41. Moss SE, Klein R, Klein BE. Ocular factors in the incidence and progression of diabetic retinopathy. Ophthalmology. 1994;101:77-83.
42. Lim LS, Lamoureux E, Saw SM, et al. Are myopic eyes less likely to have diabetic retinopathy? Ophthalmology. 2010 Mar;117(3):524-30.
43. Pierro L, Brancato R, Robino X, et al. Axial length in patients with diabetes. Retina. 1999;19(5):401-4.
44. Takahashi M, Trempe CL, Maguire K, McMeel JW. Biomicroscopic evaluation of vitreoretinal relationship in diabetic retinopathy. Arch Ophthalmol 1981;98:665-8.
45.Tolentino FI, Lee PF, Schepens CL. Biomicroscopic study of vitreous cavity in diabetic retinopathy. Arch Ophthalmol. 1966;75:238-46.
46. Davis MD. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol 1965;74:741-51.
47. Jalkh A, Takahashi M, Topilow HW, et al. Prognostic value of vitreous findings in diabetic retinopathy. Arch Ophthalmol. 1982 Mar;100(3):432-36.
48. Akiba J, Arzabe CW, Trempe CL. Posterior vitreous detachment and neovascularization in diabetic retinopathy. Ophthalmology. 1990;97:889-91.
49. Tagawa H, McMeel JW, Trempe CL. Role of the vitreous in diabetic retinopathy II. Active and inactive vitreous changes. Ophthalmology. 1986;93:1188-92.
50. Takahashi M, Trempe CL, Maguire K, McMeel JW. Vitreoretinal relationship in diabetic retinopathy. A biomicroscopic evaluation. Arch Ophthalmol. 1981 Feb;99(2):241-5.
51. Navarro A, Pournaras JA, Hoffart L, et al. Vitrectomy may prevent the occurrence of diabetic macular oedema. Acta Ophthalmol. 2010 Jun;88(4):483-5.

