A relatively new OCT metric called the choroidal vascularity index (CVI) can help determine the vascular status of the choroid in various retinal diseases. It quantifies the vessel composition of the choroid (by measuring luminal area) as a percentage of the entire structure. Higher values indicate greater vascular capacity. Many believe that evaluating the choroidal structure changes in primary open-angle glaucoma (POAG) and primary acute-closure glaucoma (PACG) is critical to understanding the different pathogeneses of these two subtypes.
 |
|
|
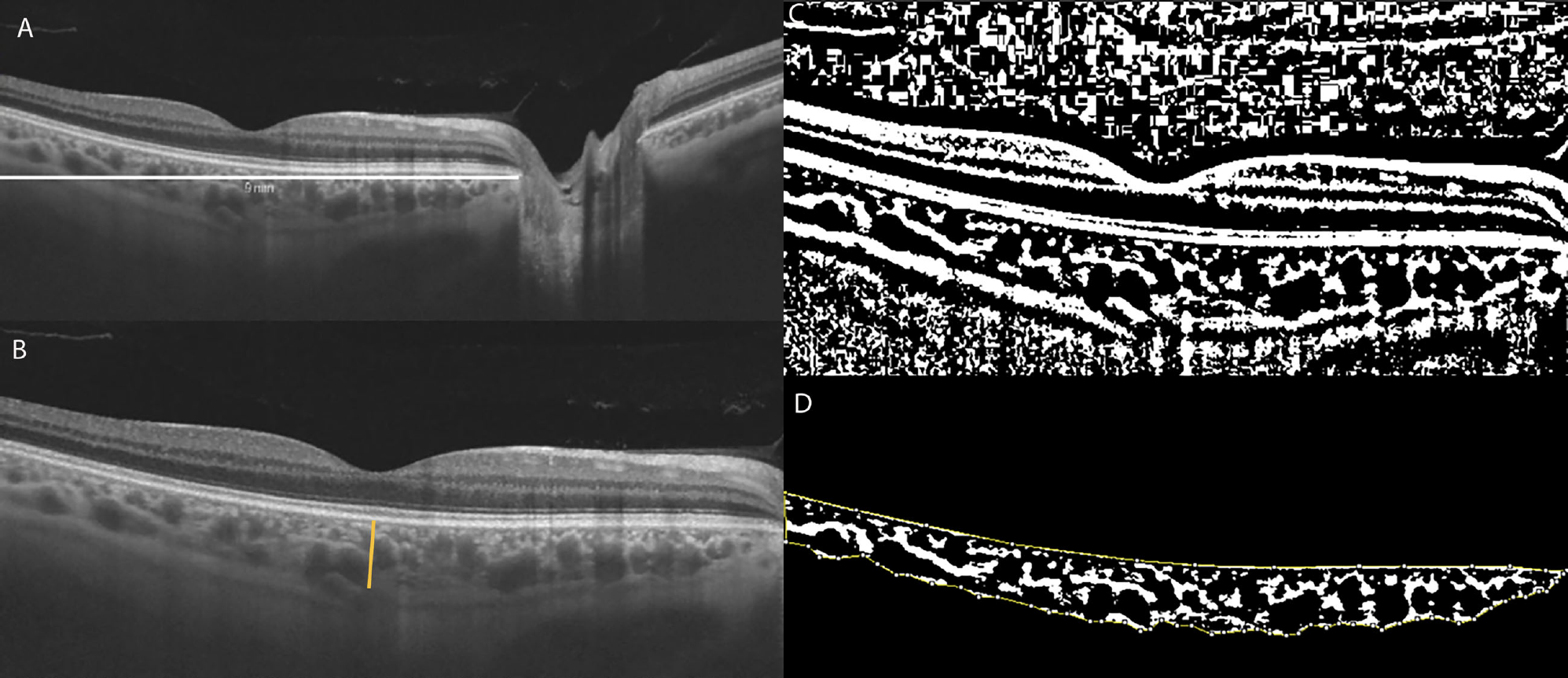
A recent study based in China has assessed structural changes in patients with POAG and PACG using the choroidal vascularity index and subfoveal choroidal thickness with enhanced-depth imaging (EDI) OCT. The research team determined that the role of the choroid may differ between POAG and PACG eyes. The choroidal vascularity index of POAG and PACG eyes was significantly lower than that of normal eyes. However, a reduced luminal area was observed mainly in eyes with POAG, and an increased stromal area was observed mainly in eyes with PACG.
A total of 171 eyes of 171 patients, comprising 69 eyes with untreated POAG, 58 eyes with untreated PACG and 44 healthy eyes, were enrolled in this study. Subfoveal choroidal thickness, luminal area, stromal area and total choroidal area were measured on EDI-OCT scans.
Eyes with POAG had abnormalities in choroidal vascularity, likely involving a reduction in the vascular area without an increase in the stromal area, which the researchers believed may contribute to the decrease in CVI. The eyes with PACG demonstrated a significantly lower choroidal vascularity index, a larger stromal area and thicker subfoveal choroidal thickness than normal eyes. This lower value indicated that PACG eyes have an increased choroidal stroma without showing a significant loss in choroidal vascular density, resulting in lower CVI.
“We hypothesize that there is potential choroidal expansion in PACG, with chronic elevated IOP and shorter axial length as possible contributing factors,” the authors wrote in their paper, which was published in Journal of Glaucoma.
A lower CVI was also significantly associated with visual field mean deviation (MD) damage in both types of glaucoma, indicating that this reduction was associated with more negative MD and glaucomatous functional damage in eyes with POAG.
The study found no significant difference in total choroidal area among the three groups, despite the significant difference in subfoveal choroidal thickness. This could be due to the latter only measuring the thickness of the foveal choroid, which may not accurately reflect the thickness of the peripheral choroid, and therefore may not fully capture changes in the total choroidal area.
“The choroidal vascularity index is very likely to be a useful indicator to further understand the different roles of the choroid in the pathogenesis of both POAG and PACG, and our results may provide valuable evidence and insights for future research,” the study concluded.
Wang D, Xiao H, Lin S, et al. Comparison of the choroid in primary open-angle and angle-closure glaucoma using optical coherence tomography. J Glaucoma. September 4, 2023. [Epub ahead of print]. |

