 |
Patients with infectious keratitis typically present urgently with sudden onset of pain, photophobia and redness. The degree of discomfort and light sensitivity may make examination challenging, and more aggressive presentations may result in permanent corneal scarring, loss of vision or even the loss of the globe. All this is enough to unsettle even a seasoned practitioner. However, with enough knowledge and careful attention to the details in presentation, successful treatment and preservation of the patient’s vision can be achieved in the majority of cases.
Epidemiology
Infectious keratitis occurs in approximately 20 to 50 per 100,000 people in the United States.1 The risk of infectious keratitis is increased following any compromise to the corneal epithelium. Principal risks include contact lens wear, trauma and ocular surface disease.2,3 Among contact lens wearers, the primary risk is overnight wear, with secondary risk coming from poor contact lens hygiene.4 As most contact lens wearers and ocular surface disease patients are under an optometrist’s care, we must be on the lookout for infectious keratitis and develop the skills to manage it.
 |
| Nocardia-induced keratitis, with several small epithelial defects and a “cracked glass” appearance. Click to enlarge. |
Clinical Presentation
Although the number of organisms capable of causing infectious keratitis is quite high, only a handful of organisms are responsible for the majority of infections. The most common bacteria present in bacterial keratitis are the gram-positive organisms of Staphylococcus and Streptococcus, which are components of the normal flora of the eyelids in adults.5 Staphylococcus species are typically listed amongst the most pervasive organisms cultured in episodes of keratitis and are the most common pathogen causing keratitis following cataract surgery, LASIK and PRK.6-9 Both Staphylococcus and Streptococcus infections are characteristically identified by a dense, round to oval, focal white infiltrate with clear margins. Although differences exist in virulence, Staphylococcus ulcerations will grow gradually over two to three days. More aggressive growth is possible, especially in cases with underlying immune compromise. Relatively speaking, Streptococcus organisms are more virulent and may expand quickly, over one to two days.
Pseudomonas is a gram-negative organism commonly associated with contact lens wear and has a more aggressive presentation than its gram-positive counterparts. Its classic presentation is an ulcer with a gray, necrotic appearance in the cornea extending out well beyond the site of excavation to involve nearly the entire cornea. Anterior chamber reaction and hypopyon are more common, and a ring infiltrate or perineural involvement may be noted.10,11 In conjunction with the increased size and depth of the infiltrate, thinning of the corneal tissue may occur quite rapidly, and Pseudomonas infections result in melting and perforation more frequently than gram-positive organisms.10 Perforation of the cornea may occur in as few as 24 hours following infection.12
Atypical mycobacteria, such as Nocardia and actinomyces, represent a smaller subset of bacterial ulcer patients. The ulcer associated with mycobacterial infection appears as a minimally excavated, focal lesion, which develops adjacent lesions, similar to the satellite lesions seen in fungal keratitis. Distinction can be made on the relatively clean appearance of the borders of the infiltrates, as opposed to the feathery edges seen in fungal infections. In addition, the appearance of the cornea immediately surrounding the lesion may have a “ground glass” or “cracked glass” appearance. These ulcers typically are slower to develop and may gradually worsen over a period of several days to weeks.13 They are also more difficult to treat, often requiring multiple medications and several weeks to months of treatment to completely eradicate.
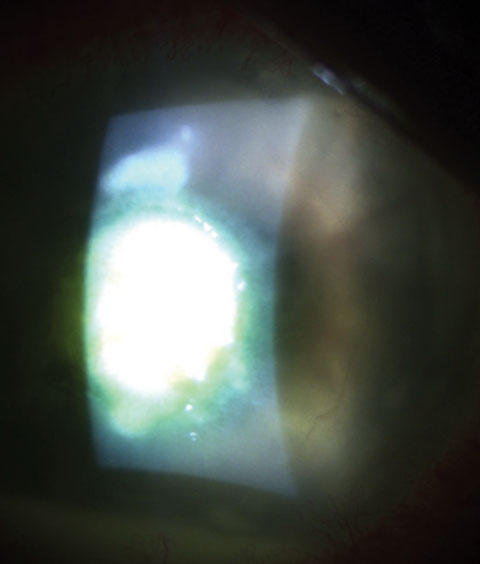 |
| A dense, round-to-oval, focal white infiltrate with clear margins is characteristic of Streptococcus infections like the one seen here. |
Culturing
Without culturing the ulcer, the identity of the bacteria can only be assumed. However, in today’s optometric practice, culturing may be performed relatively easily. Culturing should be performed with ulcers that are central, large (greater than 2mm), deep, do not show improvement with treatment, or exhibit features associated with mycobacterial, fungal or amoebic organisms.14 The first step is to establish a relationship with a microbiology reference laboratory, often located within a hospital setting. Materials needed for culturing are becoming increasingly efficient, with the advent of single-swab kits, which may be kept at room temperature for transport to the laboratory.
Treatment
In clinical practice, treatment decisions are generally empirical in nature, using broad-spectrum antibiotic therapy.15 A meta-analysis of randomized and nonrandomized studies comparing empirical treatment that use fluoroquinolones with combination therapy and fortified antibiotics shows essentially equivalent outcomes.16
Besivance (besifloxacin, Bausch + Lomb) is a chlorinated fluoroquinolone approved in the United States for bacterial conjunctivitis. It has no oral equivalent for systemic use. It has a low resistance profile, has demonstrated efficacy against gram-positive, gram-negative and anaerobic bacteria, and has been used as an adjunctive agent in mycobacteria infection.17,18 No single antibiotic provides complete coverage against all types of bacteria, so carefully monitor patients after initiation of treatment.
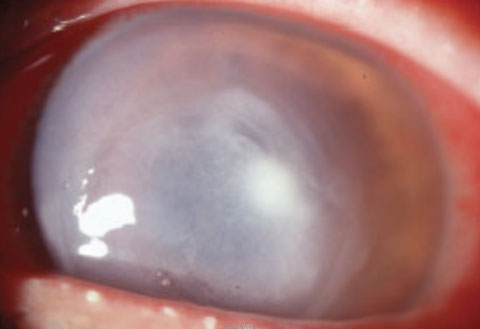 |
| The classic presentation of Pseudomonas, as seen here, involves an ulcer with a gray, necrotic appearance extending out well beyond the site of excavation to involve nearly the entire cornea. |
Empirical treatment for central or severe corneal ulcers may be performed as follows. First, a loading dose of one drop is administered every five to 15 minutes for the first 30 to 60 minutes.19 Afterwards, topical antibiotic drops are administered once every 30 to 60 minutes around the clock, with reevaluation the following day.19 The dosing may be decreased in cases of less severity to match the clinical picture.19
This dosing should be continued until the lesion shows clinical improvement, and tapered down as the keratitis resolves. No clinical benefit has been shown in tapering topical antibiotics below three to four times per day.
If the patient does not improve within 48 hours, the treatment regimen should be modified. The lack of improvement may be a result of resistance to the chosen antibiotic. In this case, culturing should be performed. As with all cases of severe infection, referral to a corneal specialist should be made in cases of impending perforation, or in cases where the keratitis is progressive or unresponsive to treatment.
The Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study published data based on more than 3,200 isolates over a five-year period. The findings indicate that methicillin-resistant organisms were noted in 42.2% of S. aureus isolates and 49.7% of coagulase-negative Staphylococcus isolates.20 More importantly, the methicillin-resistant organisms also showed high likelihood of being resistant to fluoroquinolones, aminoglycosides and macrolides, and multidrug resistance to at least three other drug classes was present in 86.8% of MRSA isolates, potentially making treatment much more difficult. Interestingly, incidence of MRSA did not increase over the five-year period.20
In Optometry’s Wheelhouse
Thanks to available knowledge regarding typical presentation patterns of these bacteria and advances in culturing techniques, successful management of bacterial keratitis is well within optometry’s set of capabilities, giving ODs yet another way to serve patients in the fight against potentially vision-threatening conditions.
Dr. Hauswirth is a consultant/speaker for Allergan, Bausch + Lomb, Biotissue and Shire Pharmaceuticals.
|
1. Pepose J, Wilhelmus K. Divergent approaches to the management of corneal ulcers. Am J Ophthalmol. 1992;114:630-2. 2. Aldebasi Y, Aly S, Ahmad M, Khan A. Incidence and risk factors of bacteria causing infectious keratitis. Saudi Med J. 2013 Nov;34(11):1156-60. 3. Ng A, To K, Choi C, et al. Predisposing factors, microbial characteristics, and clinical outcome of microbial keratitis in a tertiary centre in Hong Kong: A 10-year experience. J Ophthalmol. Available at: www.hindawi.com/journals/joph/2015/769436. Accessed: July 20, 2016. 4. Keay L, Stapleton F. Development and evaluation of evidence-based guidelines on contact lens-related microbial keratitis. Contact Lens Anterior Eye. 2008 Feb;31(1):3-12. 5. Singer T, Isenberg S, Apt L. Conjunctival anaerobic and aerobic bacterial flora in paediatric versus adult subjects. Br J Ophthalmol. 1988 Jun;72(6):448-51. 6. Lin T, Yeh L, Ma D, et al. Risk Factors and microbiological features of patients hospitalized for microbial keratitis: A 10-year study in a referral center in Taiwan. Medicine (Baltimore). 2015 Oct;94(43):e1905. 7. Sand D, She R, Shulman I, et al. Microbial keratitis in Los Angeles: the Doheny Eye Institute and the Los Angeles County Hospital Experience. Ophthalmology. 2015 May;122(5):918-24. 8. Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009 Jul-Aug;57(4):273-9. 9. Mah FS, Davidson R, Holland E, et al. ASCRS Clinical Committee. Current knowledge about and recommendations for ocular methicillin-resistant staphylococcus aureus. J Cat Refract Surg. 2014 Nov;40(11):1894-908. 10. Mascarenhas J, Srinivasan M, Chen M, et al. Differentiation of etiologic agents of bacterial keratitis from presentation characteristics. Int Ophthalmol. 2012 Dec;32(6):531-38. 11. Robbie S, Vega F, Tint N. Perineural infiltrates in Pseudomonas keratitis. J Cataract Refract Surg. 2013 Nov;39(11):1764-7. 12. McElvanney AM. Doxycycline in the management of pseudomonas cornea melting: two case reports and a review of the literature. Eye Contact Lens. 2003 Oct;29(4):258-61. 13. Garg P. Fungal, mycobacterial, and nocardia infections and the eye: an update. Eye (Lond). 2012 Feb;26(2):245-51. 14. Wilhelmus K, Liesegang T, Osato M, et al. Laboratory diagnosis of ocular infections. Washington DC: American Society for Microbiology;1994 Cumitech Series #13A. 15. Forster R. Conrad Berens Lecture. The management of infectious keratitis as we approach the 21st century. CLAO J.1998;24:175-80. 16. Hanet M, Jamart J, Chaves A. Fluoroquinolones or fortified antibiotics for bacterial keratitis: systematic review and meta-analysis of comparative studies. Can J Ophthalmol. 2012 Dec;47(6):493-9. 17. Mah F, Sanfilippo C. Besifloxacin: Efficacy and safety in treatment and prevention of ocular bacterial infections. Ophthalmol Ther. 2016 Jun;5(1):1-20. 18. Nguyen A, Hong A, Baqai J, et al. Use of topical besifloxacin in the treatment of mycobacterium chelonae Ocular Surface Infections. Cornea. 2015 Aug;34(8):967-71. 19. Feder R, McLeod S, Akpek E, et al. American Academy of Ophthalmology Bacterial Keratitis Preferred Practice Patterns - 2013. Available at: www.aao.org/preferred-practice-pattern/bacterial-keratitis-ppp--2013. Accessed: July 20, 2016.20. Asbell P, Sanfilippo C, Pillar C, et al. Antibiotic resistance among ocular pathogens in the United States: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015 Dec; 133(12):1445-54. |

