We all get our mandatory hours of continuing education to keep our optometry licenses active. How many of those hours are spent learning about evolving technology? How about the future of pharmacology? How will artificial intelligence impact eye care? A lot of changes are occurring in the field of medicine on a seemingly daily basis.
For example, more Americans are being diagnosed with diabetes mellitus every day and are in need of medical eye care to prevent sight loss. It is vital optometrists understand the pathways in which diabetic retinopathy (DR) develops, current management options and new and upcoming tools to detect DR. Will you be up-to-date on the latest technological advances and research?
Pathophysiology
Chronic hyperglycemia, thought to be the fundamental prerequisite of DR, leads to vascular changes and subsequent retinal injury and ischemia.1,2 Vascular endothelial growth factor (VEGF), expressed in response to ischemia and hypoxia, is an important factor in the development of both diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR). VEGF alters retinal capillary permeability by increasing the phosphorylation of proteins involved with tight-junctions such as zonula occludens.3,4 Since the discovery of VEGF, and subsequent reports of increased VEGF levels in eyes with PDR, investigators have extensively researched the physiological and pathological functions of VEGF.
The leading cause of vision loss in patients with DR is DME, characterized by swelling or thickening of the macula due to subretinal and intra-retinal accumulation of fluid in the macula triggered by the breakdown of the blood-retina barrier (BRB).4 Histological studies of diabetic eyes indicate that pericyte dropout from retinal capillary walls is responsible for breakdown of the inner BRB.2
New reporting supports the importance of microRNA (miRNA) in the pathogenesis of Type 2 diabetes related to insulin resistance, glucose and lipid metabolism, inflammation, diabetic nephropathy, cardiovascular disease, cartilage destruction and wound healing, hearing impairment, keratopathy, natural compound and race.5 The first study to investigate the common miRNA-146a single nucleotide polymorphism (SNP) and its relationship with diabetic microvascular complications show that this SNP may increase susceptibility to retinal damage via a pathway involved in both angiogenesis and BRB breakdown in Type 2 diabetes patients.6 Further understanding of this novel pathway causing VEGF expression and subsequent effects in the retina will undoubtedly help in the formulation of adjuvant treatment.
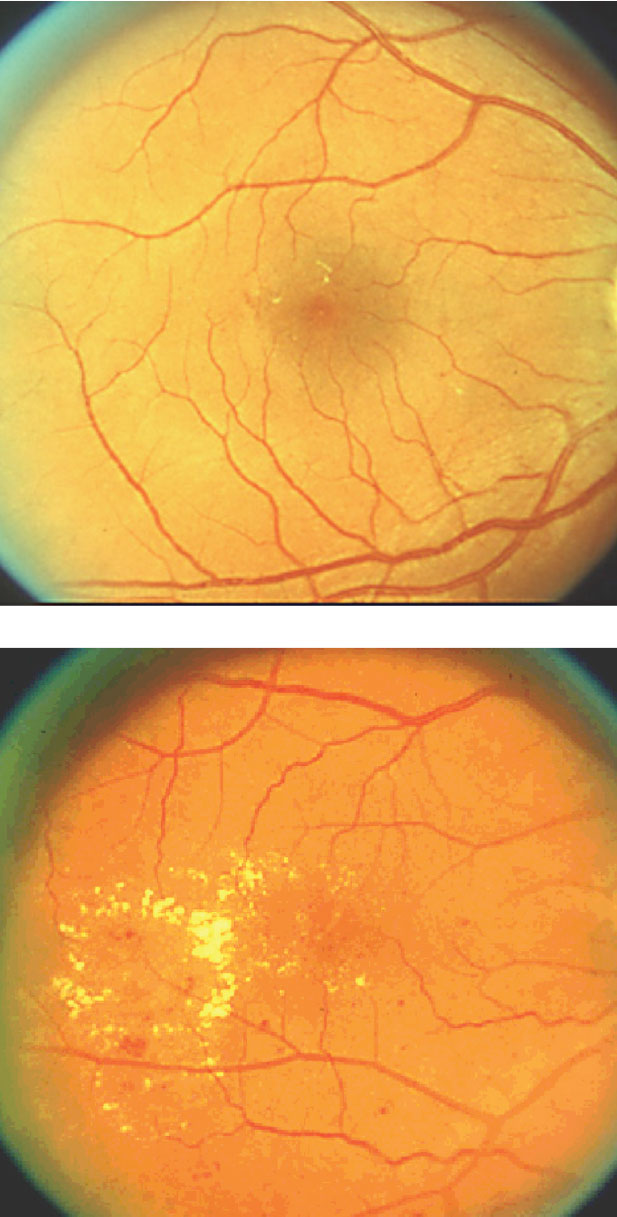
 |
| This image, obtained with fluorescein angiography, shows hyperfluorescence consistent with neovascularization. Photo: Steve Ferrucci, OD |
Laboratory Testing
Screening tests for DM include the measurement of fasting plasma glucose, a glycated hemoglobin (A1c) and a two-hour plasma glucose during an oral glucose tolerance test (OGTT). The diagnosis of diabetes is confirmed by one of the following methods:
- Fasting blood glucose level ≥126 mg/dL (7.0 mmol/L) on two separate occasions
- Random blood glucose level ≥200 mg/dL (11.1 mmol/L) if classic symptoms of diabetes (i.e. polyuria, polydipsia, weight loss, blurred vision, fatigue) are present
- OGTT with a serum blood glucose level ≥200 mg/dL (11.1 mmol/L)
- A1c measurement of 6.5% on two separate occasions.7
Diabetic Counseling
Intensive lifestyle modifications focused on weight reduction and increased activity levels should be trialed before initiating pharmacologic therapy for motivated patients with clear and modifiable contributors to hyperglycemia.8 For patients who don’t meet glycemic targets with lifestyle modifications, pharmacologic therapy with metformin may also be initiated. Additional oral medications or injectable insulin should then be considered if metformin and lifestyle modification fails.9
Early treatment for diabetes patients presenting with low (<140 mg/dl) or intermediate (140 to <180 mm/dl) fasting plasma glucose at the time of diagnosis is associated with improved glycemic control over time and ultimately decreases the risk of long-term complications including reduced risk for progression of DR.10
Currently, DR treatment is only applicable at advanced stages, and the only therapeutic strategies for early stages are a regulated control of the risk factors of DR. Methods are underway to identify patients with subclinical diabetic retinal disease and patients most prone to progressive worsening. This would allow patients, in whom intensified therapy could be prioritized, to be monitored for effectiveness of new drugs for DR.11
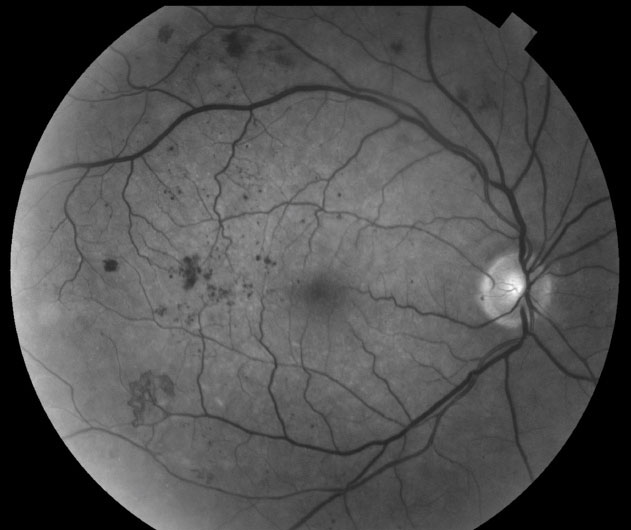
 |
| These fundus images show the difference between moderate DME (above) and severe DME. The condition is the leading cause of vision loss in DR. |
Insulin Control
Blood glucose control continues to be a challenge for physicians and patients despite pharmacologic advances. Many of the new agents available aim to improve glycemic control for longer while limiting glycemic fluctuations, hypoglycemia and weight gain.
Incretin-based therapies (IBTs) such as glucagon-like peptide-1 (GLP-1) receptor agonist and enzyme dipeptidyl peptidase (DPP-4) inhibitors offer the advantage of glycemic control without hypoglycemia and weight gain.12 This is achieved through a gradual increase in insulin secretion from glucose or food mediated through signals from the gut. GLP-1 is a natural hormone secreted in the gut that stimulates insulin release and is broken down by the enzyme DPP-4.
The pharmacologic approach to replace GLP-1 consists of either using an analogue that is resistant to DPP-4 (GLP-1 receptor agonists) or a pharmacologic agent that inhibits the activity of the enzyme DPP-4 (DPP-4 inhibitors). GLP-1 receptor agonists (liraglutide, semaglutide, lixisenatide and exenatide) increase insulin secretion by binding to the GLP-1 receptor, stimulate glucose-dependent insulin release from the pancreatic islets and decrease glucagon production, all of which lead to improved glucose control.13 These analogues have a prolonged half-life due to their variable resistance to degradation by DPP-4.
Although seemingly promising, GLP-1 receptor agonist therapy has several shortcomings, including additional training for use of injectable medications, frequent gastrointestinal side effects, high costs and the increased risk for pancreatitis.
DPP4 inhibitors (sitagliptin, saxagliptin, linagliptin and alogliptin) enhance the duration of action of endogenous GLP-1 by blocking its breakdown and provide modest glycemic control. DPP-4 inhibitors’ advantages include ease of administration using oral route, good tolerability and lack of association with weight gain or hypoglycemia. Commonly reported side effects include headache, nasopharyngitis and upper respiratory tract infection.14 The long-term safety with DPP-4 inhibitors has not been established.
Sodium-glucose cotransporter (SGLT)-2 inhibitors (canagliflozin, dapagliflozin, empagliflozin and ertugliflozin) reduce blood glucose by increasing urinary glucose excretion.15,16 SGLT-2 inhibitors are easy to use and only require once-daily oral administration. They work on all phases of glucose metabolism and modestly reduce blood pressure, weight loss and risk of hypoglycemia. The disadvantages include increased risk of genitourinary tract and fungal genital infections. SGLT-2 inhibitors should be used with caution in conjunction with other medications or comorbidities that predispose an individual to acute renal injury.
Research shows a new basal insulin analogue, degludec, is longer acting than insulin glargine and has less nocturnal hypoglycemia. Continuous glucose monitoring systems, when used together with pump therapy, also show reductions in the risk for hypoglycemia.12
Concomitant Morbidities
Several trials show strict glycemic and blood pressure control are beneficial for the prevention and progression of retinopathy.16 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study shows a reduction of retinopathy progression in an intensive glycemic therapy group.17 In patients with dyslipidemia, retinopathy progression was also slowed by fenofibrate similar to those receiving intensive glycemic treatment in the FIELD photographic sub-study. Other research shows metabolic syndrome is a strong, independent indicator of DR, even to the same extent as glycemic control.18 Moreover, research shows that PDR in Type 2 diabetes is a stronger independent correlation factor for peripheral arterial disease (PAD) than a diabetic duration of 10 years.19
A timely diagnosis of these concomitant morbidities in patients with diabetes provides valuable information regarding the risk of DR. Screening for and treating concomitant morbidities minimizes the risk of irreversible blindness from DR.
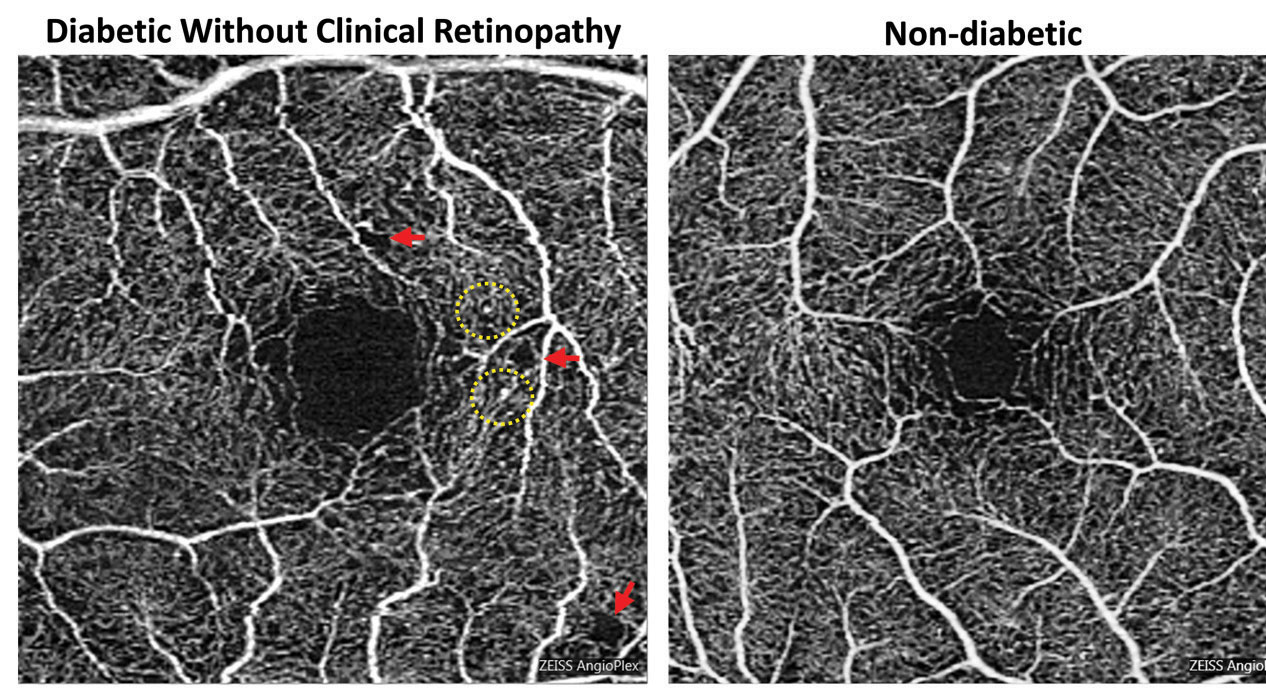 |
| Foveal enlargement and perifoveal capillary remodeling detected with OCT-A in a diabetic eye without funduscopically visible diabetic retinopathy. Red arrows point to subtle areas of capillary nonperfusion, while yellow circles highlight microaneurysms. Click image to enlarge. Photo: Carolyn Majcher, OD, and Susan Ly Johnson, OD |
Diabetic Retinal Imaging
A variety of imaging modalities can help guide the diagnosis and treatment of DR. In recent years, several technological advancements such as ultra-widefield (UWF) fundus photography, UWF fluorescein angiography (FA) and optical coherence tomography angiography (OCT-A) have been adopted by eye care practitioners for screening, evaluation and diagnosis.
Fundus photography is still a crucial tool in the education and management of DR. The standard fundus photo image captures 30˚ of the posterior pole, including the optic nerve and macula.20 These static images can easily be manipulated and magnified to detect changes in progression or improvement in diabetic eye disease.
Widefield fundus photography includes the posterior retina up to vortex vein ampullae, captures up to 105˚ field and allows for imaging of the peripheral retina.21,22 In comparison, newer UWF images capture at least four vortex ampullae in a single image and up to 200˚ of the retina.20-23 The increased use of widefield and UWF imaging for DR has begun to reveal more information about the pathology of diabetic eye disease.24,25 Using UWF retinal imaging, a 2015 study demonstrated that patients with predominately peripheral diabetic lesions had a more than fourfold greater chance of progressing to proliferative disease than those with more centrally located retinopathy.26
Fluorescein angiography (FA) can help evaluate retinal vasculature changes associated with DR. Recently, a technique was developed that improved peripheral retina imaging through combining widefield imaging with FA. UWF-FA provides valuable information about the retina, blood vessels, peripheral neovascularization and the extent of retinal nonperfusion.
Detection of nonperfused areas in the peripheral retina has given new insight into the pathogenesis of DME. Investigators hypothesized that areas of nonperfused peripheral retina are a source of VEGF, which can contribute to the formation of DME.27 Targeted retinal photocoagulation (TRP) can help reduce VEGF production, resulting decreased severity and extent of macular edema.28,29
The combination of macular laser, anti-VEGF therapy and TRP may prove to be an important treatment approach for DME while also minimizing the side effects of visual field loss from retinal laser. It should be noted that this is theoretical in nature, as no conclusive evidence shows that TRP is superior to panretinal photocoagulation (PRP).
Optical coherence tomography (OCT) uses high definition resolution to evaluate retinal anatomy and is imperative in the assessment of the macula in diabetic patients. OCT has the capability to measure and quantify macular edema and is perhaps one of the most valuable tools in managing DME. OCT helps to distinguish between center-involved or non center-involved DME, which is an important factor in determining therapeutic interventions.30
OCT-A technology enables the eye care provider to visualize vascular and morphological changes in the layers of the retina and choroid. This new technology has proved helpful in providing a better understanding of pathophysiology and treatment efficacy. We can now visualize vascular abnormalities, including areas of capillary nonperfusion, changes in the foveal avascular zone (FAZ), choriocapillaris flow, clustered capillaries, dilated capillary segments, tortuous capillaries, regions of capillary dropout, reduced capillary density, abnormal capillary loops and areas of neovascularization.31-33 One interesting OCT-A-based study found that the FAZ is larger in diabetic eyes than healthy eyes, even prior to the development of clinical DR. They further suggested that OCT-A may be useful in both screening for DM before a systemic diagnosis has been made and determining which diabetic eyes are at higher risk of DR.32
Retinal imaging has become increasingly valuable in the management of DR, as well as understanding its pathophysiology. Fundus photography provides documentation of progression or regression of DR and is commonly utilized as a screening tool. FA detects areas of retinal ischemia, vascular leakage and macular edema. OCT is important for the detection and management of macular edema. Multimodal data are being integrated by artificial intelligence-based systems in the screening and management of DR.34
Treatments
It is no secret that anti-VEGF therapies have become the mainstay of treatment for many patients with DME. Anti-VEGF therapy is proving to be superior to laser treatments, which are associated with resultant tissue damage and vision loss.35 Intravitreal Lucentis (ranibizumab, Genentech) and Eyelea (aflibercept, Regeneron) are FDA-approved therapies often used for DME, whereas intravitreal Avastin (bevacizumab, Genentech) is administrated off-label.36,37 The RISE, RIDE, VIVID and VISTA studies all support anti-VEGF agents over laser and early intervention in patients with DME to maximize potential visual acuity.38,39
In a two-year randomized clinical trial, Protocol T compared three anti-VEGF agents (ranibizumab 0.5mg, aflibercept 2.0mg and bevacizumab 1.25mg) for the treatment of DME.40 After two years of follow up, patients with an initial BCVA of 20/50 or better achieved similar visual improvement with all three drugs. Aflibercept was associated with the greatest reduction in mean central macular subfield thickness on OCT. Patients presenting with BCVA of 20/50 or worse had the greatest visual improvement at one year with aflibercept; however, the results diminished and were no longer statistically significant at the two-year mark.
For the treatment of center-involved DME, there may be reason to prefer one anti-VEGF over another; however, all three agents investigated in Protocol T were beneficial for most patients.
The treatment paradigm for PDR is also starting to shift in favor of injectable therapeutic agents. The Diabetic Retinopathy Clinical Research Network conducted a major randomized clinical trial, Protocol S, for the pharmacologic treatment of PDR using ranibizumab compared with PRP.41,42 The two-year findings of Protocol S revealed that ranibizumab was non-inferior to PRP with a mean gain of +2.8 letters vs. +0.2 letters in the PRP group.43 Although these results were quite promising, the role of ocular anti-VEGF therapy for PDR is less clear. Patients may require indefinite injections and monitoring due to the limited half-life of these drugs.
The CLARITY study is the first randomized controlled trial of intravitreal aflibercept in PDR. The results provide substantial evidence that the visual outcome in those with active PDR at one year with aflibercept is superior to PRP.44
The ongoing PANORMA study is evaluating aflibercept injections in patients with moderate and severe non-proliferative diabetic retinopathy (NPDR). Results are impressive with patients exhibiting a two-step or greater improvement based on the Diabetic Retinopathy Severity Scale (DRSS).45 The implications of this study are significant as there is a realistic potential for many patients to opt for earlier pharmacological intervention if worsening stages of NPDR or PDR can be prevented.
It should be noted that anti-VEGF agents have gained favorability for treating DME and PDR over the past decade; however, focal/grid photocoagulation and PRP remain the gold standard treatments for DME and PDR, respectively.
Artificial Intelligence
The infusion of artificial intelligence (AI) into eye care is progressing every day. The field of AI has recently experienced significant advancements in image recognition due to a technique called deep learning (DL) which, when applied to current imaging, results in improved diabetic eye disease recognition and identification of progression. One study applied DL to retinal photography to create an algorithm to automatically detect the presence of DR and DME using 128,175 macular-centered retinal images. Results show astounding sensitivity and specificity for referable DR more than 90%.46 DL has also been applied to macular OCT scans. A 2018 study shows that DL achieves excellent accuracy in the detection of retinal fluid across several macular diseases and OCT devices. Furthermore, AI results were in high concordance with manual expert clinical assessments.47,48
Such AI devices and software provide a quantitative and qualitative analysis without the need for manual interpretation. This opens the realistic potential of AI-based programs for use outside the optometrists and ophthalmologists’ office. The integration of AI can be expected to radically change clinical practice with more people getting screened for retinopathy remotely. AI will continue to expand and become an auxiliary component and useful tool in DR screening, but AI cannot replace the role of eye care providers in clinical diagnosis and management. Although there are challenges ahead, AI will likely impact optometry practices in the coming decades by providing increased efficiency, reducing overall costs, improving access to care and ultimately reducing the strain on our current healthcare system.
Dr. Gentry practices at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Mo.
Dr. Ho practices at the Central Texas Veterans Health Care System Austin Outpatient Clinic in Austin, Texas.
Dr. Zimbalist practices at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Mo.
Dr. O’Brien is a 2019 graduate of the University of Missouri-St. Louis College of Optometry.
1. Kusuhara S, Fukushima Y, Ogura S, et al. Pathophysiology of diabetic retinopathy: The old and the new. Diabetes Metab J. 2018;42(5):364-76. 2. McCulloch D, Robertson R. Pathogenesis of type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/pathogenesis-of-type-2-diabetes-mellitus. Accessed March 8, 2019. 3. Gupta N, Mansoor S, Sharma A, et al. Diabetic retinopathy and VEGF. Open Ophthalmol J. 2013;7:4-10. 4. Wang W, Lo A. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol Sci. 2018;19(11):E1816. 5. Miao C, Zhang G, Xie Z, Chang J. MicroRNAs in the pathogenesis of type 2 diabetes: new research progress and future direction. Can J Physiol Pharmacol 2018;96(2):103-12. 6. Kaidonis G, Gillies M, Abhary S, et al. A single-nucleotide polymorphism in the MicroRNA-146a gene is associated with diabetic nephropathy and sight-threatening diabetic retinopathy in Caucasion patients. Acta Diabetol. 2016;53:643-650. 7. McCulloch D, Hayward R. Screening for type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/screening-for-type-2-diabetes-mellitus. Accessed March 7, 2019. 8. Wexler D. Initial management of blood glucose in adults with type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/initial-management-of-blood-glucose-in-adults-with-type-2-diabetes-mellitus. March 21, 2019. Accessed May 20, 2019. 9. McCulloch D. Oveview of medical care in adults with diabetes mellitus. UpToDate. www.uptodate.com/contents/overview-of-medical-care-in-adults-with-diabetes-mellitus. March 21, 2019. Accessed May 20, 2019. 10. Colagiuri S, Cull C, Holman R, UKPDS Group. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: U.K. prospective diabetes study 61. Diabetes Care 2002; 25:1410. 11. Simó-Servat O, Simó R, Hernández C. Circulating biomarkers of diabetic retinopathy: an overview based on physiopathology. J Diabetes Res. 2016;2016:5263798. 12. Fonseca V. New developments in diabetes management: medications of the 21st century. Clinical Therapeutics 2014 April;36(4):477-84. 13. Dugan K, DeSantis A. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/glucagon-like-peptide-1-receptor-agonists-for-the-treatment-of-type-2-diabetes-mellitus. January 14, 2019. Accessed March 8, 2019. 14. Dugan K, DeSantis A. Dipeptidyl peptidase-4 (DPP-4) inhibitors for the treatment of type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/dipeptidyl-peptidase-4-dpp-4-inhibitors-for-the-treatment-of-type-2-diabetes-mellitus. January 22, 2019. Accessed March 10, 2019. 15. De Santis A. Sodium-glucose co-transporter 2 inhibitors for the treatment of type 2 diabetes mellitus. UpToDate. Sodium-glucose co-transporter 2 inhibitors for the treatment of type 2 diabetes mellitus. UpToDate. December 10, 2018. Accessed March 10, 2019. 16. Mohamed Q, Gillies M, Wong T: Management of diabetic retinopathy: a systematic review. JAMA 2007;298(8):902-16. 17. Chew E, Davis M, Danis R, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the ACCORD eye study. Ophthalmology. 2014;121(12): 2443-51. 18. Gao L, Xin Z, Yuan M, et al. High prevalence of diabetic retinopathy in diabetic patients concomitant with metabolic syndrome. PLoS ONE. 2016;11(1):e0145293. 19. Chen Y, Wang Y, Zhao D, et al. High prevalence of lower extremity peripheral artery disease in type 2 diabetes patients with proliferative diabetic retinopathy. PLoS One. 2015 Mar; 10(3):e0122022. 20. Salz DA, Witkin AJ. Imaging diabetic retinopathy. Middle East Afr J Ophthalmol. 2015;22(2):145-150. 21. Patrick JS, Tyler ME. Fundus photography overview. In: Ophthalmic Photography: Retinal Photography, Angiography, and Electronic Imaging, 2nd ed. New York, NY: Butterworth-Heinemann Medical; 2001. 22. Bethke W. The devil’s in the distant details. Review of Ophthalmol. 2014;20(8):26-28. 23. Kiss S. Going ultra-wide. Retina Today. 2018;13(10)46-8. 24. Brown K, Sewell JM, Trempe C, et al. Comparison of image-assisted versus traditional fundus examination. Eye and Brain. 2013;2013(5):1-8. 25. Wessel M, Aaker G, Parlitsis G, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785-91. 26. Silva P, Cavallerano J, Haddad N, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmol. 2015;122(5):949-56. 27. Oliver C, Schwartz S. Ultra-widefield fluorescein angiography. In: Arevalo JF, ed. Retinal Angiography and Optical Coherence Tomography, 1st ed. New York, NY: Springer Science and Business Media, LLC; 2009:407-417. 28. Reddy S, Hu A, Schwartz SD. Ultra-wide field fluorescein angiography guided targeted retinal photocoagulation (TRP). Semin Ophthalmol. 2009;24(1):9-14. 29. Muquit M, Marcellino G, Henson D, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophtalmol. 2013;91(3):251-8. 30. Cunha-Vaz J, Coscas G. Diagnosis of macular edema. Ophtahlmologica. 2010;224(suppl 1):2-7. 31. de Carlo TE, Chin AT, Bonini-Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35(11):2364-70. 32. Takase N, Nozaki M, Kato A, et al. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35(11):2377-83. 33. Choi W, Waheed NK, Moult EM, et al. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina. 2017;37(1):11-21. 34. Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, et al. Artificial intelligence in retina. Prog Retin Eye Res November 2018;67:1-29. 35. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. 36. Ferrara N., Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15(6):385-403. 37. van Asten F, Michels CTJ, Hoyng CB, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration: A cost-effectiveness analysis from a societal perspective. PloS ONE 2018;13(5):1-14. 38. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from VISTA and VIVID studies. Ophthalmol. 2015;122(10):2044-52 39. Brown D, Nguyen Q, Marcus D, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmol. 2013;120(10):2013-22. 40. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-203. 41. Wells J, Glassman A, Ayala A, et al. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmol. 2016;123(6):1351-9. 42. Heier J, Bresler N, Avery R, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: Extrapolation of data to clinical practice. JAMA Ophthalmol. 2016;134(1):95-9. 43. Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross JC, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial JAMA. 2015;314(20):2137-46. 44. Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-203. 45. Eylea (aflibercept) injection improves diabetic retinopathy and reduces vision-threatening complications in phase 3 trial. Up to date. www.biospace.com/article/releases/eylea-aflibercept-injection-improves-diabetic-retinopathy-and-reduces-vision-threatening-complications-in-phase-3-trial/. 46. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of DR in retinal fundus photographs. JAMA. December 13, 2016. jamanetwork.com/journals/jama/fullarticle/2588763. Accessed May 20, 2019. 47. Schlegl T, Waldstein S, Bogunovic H, et al. Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmol. 2018;125(4):549-58. 48. Walton OB 4th, Garoon RB, Weng CY, et al. Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol. 2016;134(2):204-9. |

