Download PDFClick here to download a PDF of this article from our July issue. |
A condition as pervasive as dry eye is sustained by the rhythms of modern life. Our reliance on digital screens, our poor sleep habits and dietary choices, the harsh climates we expose our eyes to, the medications we take as well as the conditions they treat, the cosmetics and contact lenses we wear—all these are on-ramps for the family of conditions we call ocular surface disease, chief among them dry eye.
Against this backdrop, in late 2020 the Tear Film and Ocular Surface Society (TFOS) created a new workshop comprised of 158 clinical and academic researchers from 38 countries to systematically evaluate the scientific literature on how a patient’s day-to-day experiences—some volitional, some not—give rise to dry eye, digital eye strain, microbial keratitis, pterygia and a host of other conditions.
The TFOS Lifestyle Workshop, as it’s called, is a collection of 10 reports just published in The Ocular Surface that will surely be the most definitive statement attempted thus far on these often-unavoidable triggers for ocular surface disease. TFOS also released a series of videos in early May that sketch out the key insights of the workshop’s eight subcommittees.
Below, we’ll walk you through this treasure drove of data using quotes from the video presentations, journal articles and original interviews conducted with several participants for added context.
“People are exposing themselves to so many ocular risk factors nowadays,” says Dr. Jennifer Craig, a professor at New Zealand’s University of Auckland and Chair of the TFOS Lifestyle Workshop. With the ubiquity of digital screens and the growing popularity of myopia management interventions, this is starting at such a young age; furthermore, people are living longer. “If we’re causing damage at a younger and younger age, we’ve got a lot of years to live with the consequences of that damage.” Here’s what Dr. Craig and her colleagues advocate in response.
Contact Lens Wear
Patients who wear contact lenses consistently point to convenience and cosmesis as motivators. In literature reviewed by this subcommittee, such individuals reported better quality of life than those wearing glasses among kids, adults and elderly alike, all citing better vision quality and comfort leading to increased satisfaction.
Despite the obvious advantages, especially when playing sports, contact lenses may contribute to or exacerbate dry eye. However, the rate at which that occurs may differ for experienced vs. new lens users. While the contact lens discontinuation rate remains about 25%, the reasons why differ. For new wearers, their most cited reasons were discomfort (35%), lens handling (33%) and loss of interest. However, experienced wearers most often cited ocular discomfort, followed by inconvenience, which may be partially due to said discomfort. Although not a precise complaint, ocular discomfort is often caused by feelings of dryness. This is corroborated by evidence that dropouts have shown shorter tear breakup time, meibomian gland plugging, worse meibum quality and greater likelihood of being diagnosed with dry eye.1
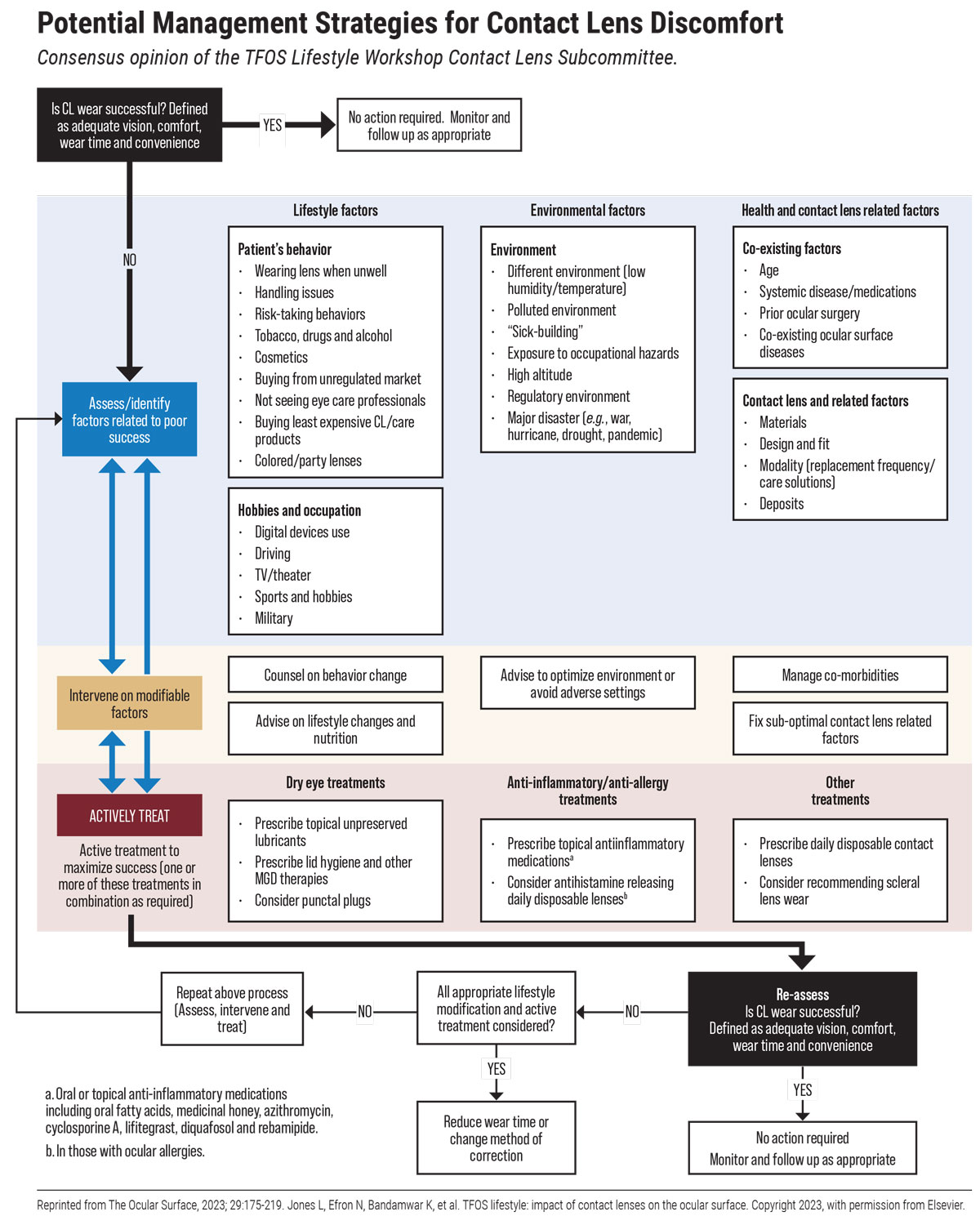
Going into greater detail, this subcommittee identified nine areas of lifestyle choices regarding contact lens use and wear that could affect dry eye and other ocular surface effects.
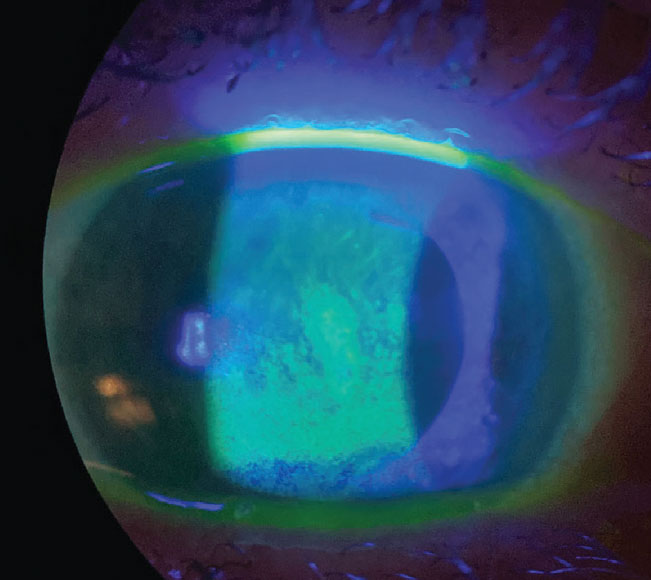 |
|
An example of dry eye compromising the ocular surface, seen with sodium fluorescein staining. Photo: Pam Theriot, OD. Click image to enlarge. |
The first category as it relates to contact lenses is how lenses are obtained. “Purchasing lenses from unregulated outlets in particular is a concern for patients wearing cosmetic lenses or sort of party type lenses that certainly impact the risk of microbial keratitis. There’s very good evidence around that fact, and some of it relates to lenses being shared with friends,” states Dr. Lyndon Jones, of the School of Optometry and Vision Science at University of Waterloo, Canada and the primary author of this report.
Age is a significant risk factor in ocular surface health and continued contact lens success. Children have fewer complications than adults, likely due to parents managing their wear. As the ocular surface worsens with age, so does the contact lens complication rate, to some extent. Complications are highest in young adults, attributed to comparatively poorer adherence to proper hygiene measures. As such, the subcommittee recommends that safety education and continual connection with an eyecare provider are integral parts of ensuring hygiene compliance.
The COVID-19 pandemic brought with it unforeseen complications in all aspects of life and will be discussed in other subsections of this article. However, as it related to contacts, the subcommittee highlighted that there is a great lack of existing evidence for what patients should do when unwell in general, but especially with viral upper respiratory tract infections such as COVID. Long COVID has been linked to corneal epithelial nerve loss and an increase in immune cell density of the cornea, potentially creating long-term impediments to contact lens wear. Furthermore, contact lens wearers who have a lower quality precorneal tear film may experience worsened dry eye symptoms from mask-associated dry eye. Some evidence points to illness mapping onto corneal inflammatory events associated with contact lens wear, prompting Dr. Jones to suggest affected patients “refrain from contact lens wear until they are fully better.”
Despite this potential link, COVID itself did not end up directly impacting typical contact lens–associated risk factors; instead, performance may have been lessened from mask-associated dry eye, increased screen time and hand sanitizer use.
Other health concerns such as thyroid eye disease and diabetes, both in their ocular manifestations and the medications used, can complicate lens wear. Problems can also arise after cosmetic or refractive surgeries due to potential corneal shape changes, eyelid configuration or contour modifications.
Vision and comfort decline as the tear film thins, leading environmental factors like decreased temperature and humidity to increase dryness of the eyes through a less stable tear film and subsequent tear film evaporation. Also having the ability to impact lens wear negatively are low humidity, high altitude, pollution, wind, dust and fumes.
Reliance on digital devices results in a compromised environment of the ocular surface and exacerbates existing difficulties in contact lens wear, as reduced blink frequency and blink amplitude lead to complaints of eye strain, dry eyes, brining, irritation and blurry vision. The contact lens subcommittee report stresses that it is important to remember that full correction is needed for contact lens wearers to achieve maximal performance, especially when heavily using digital devices. Multifocal performance is better than with monovision lenses and low-level astigmatism should be fully corrected for as well.
Lens wearers with industrial occupations should be cautious of foreign bodies, chemical fumes, vapors and aerosol droplets, as these can pose problems. Flying is also well-known to decrease lens performance from the low humidity and resultant dry environment.
Interestingly, a high risk-taking personality was found to be a better predictor of compliance than factors of age, sex or practitioner perception, with those that possess this trait being much less compliant than their counterparts.
The report notes that 99% of wearers admit they have engaged in at least one hygienic risk behavior, so it’s no wonder microbial keratitis and other contact complications account for one million US doctor visits each year. Risk increases with sleeping in lenses, which is the single largest risk factor for serious complications. Others include topping off lens solutions in cases, using tap water to store lenses and infrequent cleaning or replacing of lenses and cases.
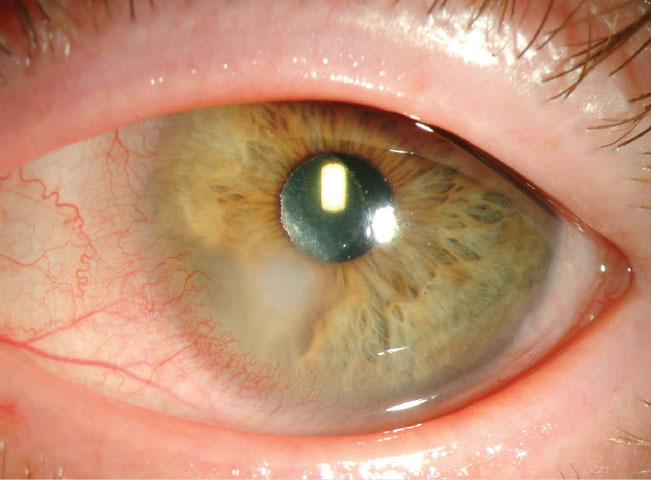 |
|
An example of a Pseudomonas infection, which can be far more commonly found in nonadherent contact lens patients. Photo: Christine Sindt, OD. Click image to enlarge. |
Other complications have been reported in contact lens wearers after smoking tobacco, marijuana or e-cigarettes and those under the influence of drugs or excessive alcohol consumption. These included increased inflammatory and infective rates and decreased performance.
Daily disposables are more convenient for patients since there is no upkeep; not surprisingly, they have the highest compliance with lens replacement and the lowest complication rates. Wearers also have fewer inflammatory complications and less severe cases of microbial keratitis. However, patients who inappropriately reuse daily disposables are actually most at risk of developing complications, since the practitioner doesn’t go over proper hygiene guidance, as the lenses are meant to be single-use products.
Perhaps related to patients reusing dailies is that they “don’t think about contact lenses as being medical devices,” Dr. Jones points out. “They think of them as being a commodity product, so I think the big thing for me is to give practitioners the opportunity to be able to get that message across to patients that lenses are safe—unless you don’t do what you’re supposed to. In which case, the outcome can be not so nice.” This could also have been exacerbated by what he explains as the pandemic causing patients to avoid frequent follow-up to save time and costs, but ultimately contributing to reduced satisfaction with lenses and increased risk.
The systematic review covered by this subcommittee looked at the association of lifestyle factors and soft contact lens dropout. They found, not surprisingly, that the most common reason for dropout was lens discomfort. The most common reason for dropout among presbyopes using multifocals was for vision quality. However, the most common general reason suggests contacts are contributing to concerns of dry eye symptoms in a substantial number of patients.
 |
| Click image to enlarge. |
Use of Cosmetic Products
Few consumer products are as problematic as cosmetics—used worldwide and all throughout history but defined, manufactured and regulated in very inconsistent ways. As the eye makeup industry alone is valued at $15.5 billion, the prominence with which these cosmetics are used should prompt more stringent safety protocols. Unfortunately, this is not the reality of the industry right now, with TFOS outlining that a “number of these ingredients can act as allergens, carcinogens, endocrine disruptors, immunosuppressants, irritants, mutagens, toxins and/or tumor promoters and may damage the ocular surface and adnexa.”2
In the US, the FDA estimates 12,500 chemicals are used in cosmetics, but less than 20% of those have been reviewed for safety.2 The US is much more lax than European countries in regulating cosmetic manufacturing; the US has only banned 11 chemicals for toxicity, while the EU has banned or restricted over 1,300.
Covered in the report are the most deleterious offenders known so far that have made their way into cosmetic products, including benzalkonium chloride, chlorphenesin, formaldehyde-releasing compounds, parabens, phenoxyethanol, phthalates, prostaglandin analogues, retinoids (vitamin A metabolites), salicylic acid and tea tree oil (terpinen-4-ol).
Benzalkonium chloride may induce tear film instability, goblet cell loss, conjunctival squamous metaplasia, cell apoptosis and disrupted corneal epithelial barrier. The preservative can result in irritating, burning, itching, foreign body sensation, conjunctival hyperemia, blepharitis, meibomian gland loss, DED, glaucoma surgery failure and anaphylaxis. It’s not surprising, based on these manifestations, that the compound has been found toxic to corneal, conjunctival and meibomian gland epithelial cells in vitro at levels much lower than those approved for eye makeup use.
Formaldehyde-releasing compounds and parabens are likewise toxic to corneal, conjunctival and meibomian gland epithelial cells. They can cause damage due to mutagenic, carcinogenic and pro-allergenic properties. Parabens, used in over 22,000 US cosmetics, act as allergens and endocrine disruptors while also possessing estrogen potency and anti-androgen activity, with the most common to look out for being methylparaben and ethylparaben. Through these properties, these compounds may increase malignancy risk.
Phthalates are a compound used in cosmetics as solvents in removers or fragrances and are sometimes found as plasticizers in packing materials that can leach into cosmetics unintentionally. They, like parabens, are linked to endocrine disruption and anti-androgen activity as well as reproductive disorders, hepatotoxicity, neurotoxicity and more. They negatively impact corneal endothelial cells’ growth and viability. Consequently, dibutyl phthalates have been banned in Europe for cosmetic use, but still exist in many US products.
Tea tree oil is another endocrine disruptor with anti-androgen activity, specifically linked to promoting meibomian gland dysfunction. However, unlike the others, it does possess some valid medical uses, like in relieving Demodex infestations. However, terpinen-4-ol should be used judiciously since it may contribute to antibiotic resistance in human pathogens, the report says. It may also induce gynecomastia in boys and has been found to kill all meibomian gland and corneal and conjunctival epithelial cells at 1% concentration after only 90 minutes in vitro.
Other ingredients often causing ocular discomfort are castor oil, fragrances, gold, heavy metals and talc. These ingredients are often linked to contact dermatitis. Ingredients also causing ocular discomfort but for different reasons are prostaglandin analogs, retinoids and salicylic acid. While retinoids and salicylic acid are not typically used in cosmetics, being placed closely on the skin near the eyes can cause meibomian gland changes and ocular surface toxicity, respectively.2
It’s not just which kinds of makeup are applied that can cause problems, but how. Sharing eye makeup can introduce bacterial, viral or Demodex transmission. Repeatedly using a single cosmetic can introduce microbes into containers; this has been corroborated by 79% of used mascaras testing positive for Staphylococcus aureus and 13% for Pseudomonas aeruginosa. Even further, contamination rates are linked to amount of use, product age and the number of users.
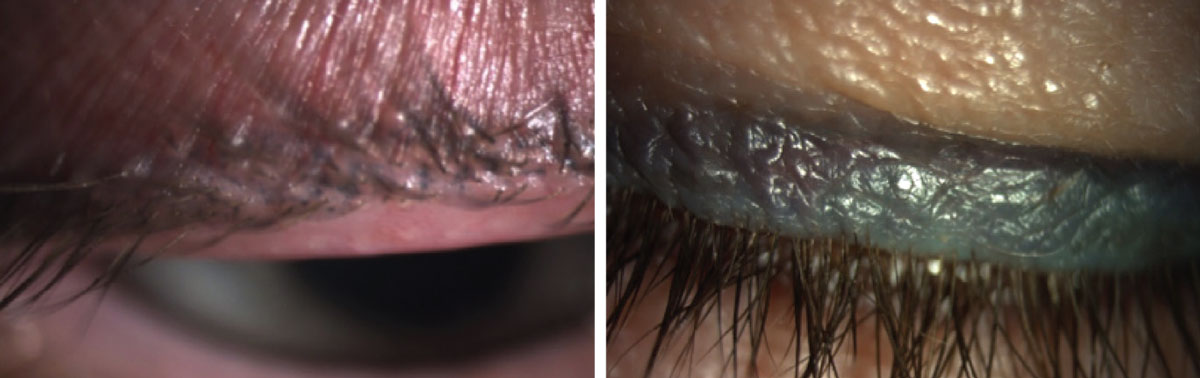 |
|
Inappropriate use of permanent makeup. Both patients have dry eye with MGD. Photos: Tracy Doll, OD. Click image to enlarge. |
Different cosmetics applied on or near the eye vary widely in observed adverse effects. Mascara can migrate to the ocular surface itself through blinking, can block meibomian glands when applied to the mucocutaneous junction of the lid margin and can obstruct the nasolacrimal duct and canaliculi as well as cause contact dermatitis from ingredients. Eyeliner similarly can predispose cosmetics to migrate to the ocular surface when applied to the mucocutaneous junction and contribute to meibomian gland dysfunction. Eye shadow contributes to irritation and eye primers to allergic reactions.2
The brushes and sponges used to apply makeup often harbor microbial growth through skin oil, debris and moisture. One recommendation is to use hypochlorous acid wipes as a cleaning solution or makeup remover, since it possesses antimicrobial properties. By contrast, the often-used micellar makeup removers can migrate to under the eyelids and increase tear film evaporation or cause decreased tear stability, while oil-free surfactant removers can cause eyelid irritation.2
This subcommittee’s systematic review also tackled whether eyelash growth products are linked to ocular surface disease signs or symptoms; based on the current literature, it was unable to come up with clear conclusions.
However, the subcommittee was able to provide some concrete recommendations for the cosmetics industry and/or other stakeholders like the FDA:
- Provide information about a cosmetic’s function, toxicity, indications, contraindications, durability and expiration date, as well as concentration.
- Perform well-controlled, high-quality studies to examine acute and chronic effects of eye cosmetic ingredients and procedures on the ocular surface and adnexa.
- Develop guidelines to assess safety and tolerability of eye cosmetic products.
- Establish more stringent and rigorous oversight of eye makeup industry.
- Develop standardized and universally accepted definitions of the words ‘clean’ and ‘natural’ as they relate to cosmetics.
- Educate eyecare providers and consumers about risks associated with ingredients in eye cosmetic products.
Digital Device Use
It has been widely recognized that our dependence on modern technology is likely causing all sorts of problems, from cognitive changes in children to sleep problems and much more. While digital eye strain is one such effect, this subcommittee refreshingly does not try to offer admonitions to lower one’s digital consumption, recognizing that it’s just not feasible in today’s world.
Instead, this report covers how digital eye strain is often exacerbated by other underlying conditions and offers solutions as they relate to those. First, though, the group had to define digital eye strain: the development or exacerbation of recurrent ocular symptoms and/or signs, related specifically to digital device screen viewing.
This report looked at many different types of displays and their characteristics, such as size, resolution, refresh rate and viewing distance, all of which can have an effect on the ocular surface.
Diagnosing digital eye strain comes with challenges. Two of the most popular questionnaires used in patient screening—the Computer Vision Syndrome Questionnaire (CVS-Q) and the Computer-Vision Symptom Scale (CVSS17)—fail to address whether symptoms are also experienced outside of digital device use. They look at frequency and severity of symptoms, but “there is no criteria to link questionnaires specifically to digital device use and many require only one symptom to be reported, hence we have a very high prevalence,” notes subcommittee chair James Wolffsohn, FCOptom, PhD, head of the School of Optometry at Aston University, Birmingham, UK. Reported rates of digital eye strain vary widely, from 32% to 98%, depending on diagnostic criteria and occupation.
Symptoms on the questionnaires include burning, eye pain, headache, redness, photophobia, tearing, repeated/frequent blinking, heavy eyelids, itching, blurred vision, double vision, eye strain and foreign body sensation, many of which could be indications of other underlying problems.
Thus, the subcommittee concluded that “currently there is no robust algorithm to diagnose digital eye strain and many people ‘diagnosed’ with digital eye strain probably have dry eye disease, uncorrected/not fully corrected refractive error and/or a binocular vision anomaly which have their own diagnostic criteria and established evidence-based management strategies.”3
The subcommittee encourages doctors to elicit reports of recurrent ocular symptoms (and possibly signs) specifically when using digital devices. It is important to check with patients that any increase in symptoms or signs while using digital devices is not also occurring during non-digital tasks.
Reasons for digital eye strain occurring are mainly due to mechanisms of blink abnormalities that cause ocular surface and tear film alterations, the report notes. This may be caused by underlying vision or accommodation deficiencies as well as oculomotor function that can induce visual disturbances like asthenopia, blurred vision and focusing and accommodative difficulties.
 |
| While device use can lead to digital eye strain, underlying conditions need to be checked in order to determine if the strain is a symptom originating elsewhere. Photo: Getty Images. Click image to enlarge. |
The report also covered possible management strategies that could improve symptoms of digital eye strain.
“Oral omega-3 supplementation does seem to have a beneficial effect on digital eye strain,” Dr. Wolffsohn says. “We know omega-3 is good for ocular health in terms of anti-inflammatory effects, and as we know that ocular discomfort generally has an inflammatory component, this may explain its benefit.”
Likely effective management strategies include reminders created through software. These can pop up on a computer or phone screen to notify the user to take rest breaks, provide blink reminders or adhere to the 20/20/20 rule. Other likely strategies are artificial tears, probiotics, eyelid warming, humidifiers and using e-paper devices (like a Kindle) when possible. Notably, however, the blue-light blocking lenses that have become popular in some circles were shown to be ineffective—or, rather, to lack solid scientific evidence of support for recommended use—as was antioxidant supplementation.3 The report advises practitioners not to recommend patients invest in blue-light blocking lenses, as they are often costly and have thus far failed to show documented clinical benefits.
Other options center on the task itself, with advice on altering device settings or the type of device being used. It has been found that digital eye strain usually occurs around the four- to five-hour mark, so working for that long or extended past this frame may exacerbate symptoms, explains Dr. Wolffsohn. As well, digital eye strain may be made worse through cognitively demanding material.3
A few studies have found more demanding tasks to cause users to reduce their blink rate, since longer fixation is needed to perform said task. Interestingly, this could be a subconscious mechanism to attempt to stay focused by avoiding interruptions. As such, interspersing demanding work with passive activity may prove a good way to tamp down eye strain. Device settings can be changed to help with symptoms, including increasing font size and changing the type to something more easily readable. Bypassing small devices for larger ones and increasing view distance may also provide some benefit, as may using circularly polarized light-emitting displays.3
Clarifying what is actually effective will hopefully combat the productivity lost through this condition and improve quality of life.
Medications & Elective Procedures
Just as with cosmetics use, elective (or semi-elective) procedures and medications are meant to enhance quality of life. However, no surgical or medical intervention is without potential side effects, and those impacting the ocular surface are certainly no exception.
Medications can cause ocular surface disease through toxicity of preservatives, ingredients or excipients like surfactants, co-solubilizers and preservative aids, pH or tonicity of the formula or from drug overuse.
Benzalkonium chloride is the main preservative known to cause toxic effects, outlined in the Cosmetics section. Preservatives can also cause allergic and immune-inflammatory effects. Artificial tears, gels and ointments used for dry eye, while offering benefits, can cause their own adverse reactions. If opting for preserved agents, ‘soft’ or ‘disappearing’ preservatives like Polyquad, sodium perborate, Purite and SofZia show lower cytotoxic effects to the ocular surface than benzalkonium chloride does, according to the report.4
Alternative medicines like aloe vera and manuka honey have both been recommended for dry eye. Note that aloe can result in ocular allergies, redness, irritation or burning, and manuka honey or honey eye drops or gels can produce conjunctival inflammation and should not be used if patients have an allergy to bee products.
Antihistamines, mast cell stabilizers and dual-acting drugs are all anti-allergic therapies known to cause a wide range of negative ocular effects, as can topical alpha-adrenergic receptor agonists and NSAIDs. Antihistamines can have mild side effects. Azelastine and emedastine can cause ocular irritation and dysgeusia, and emedastine can also result in burning, stinging, itching, dry eye, epiphora and visual disturbances. Systemic antihistamines may be more of an issue, as they can decrease aqueous and mucin production from lacrimal glands and goblet cells and induce lacrimal gland vasoconstriction, causing decreased tear production to lead to dry eye disease. Topical versions are preferred when possible since their adverse effects are lessened.4
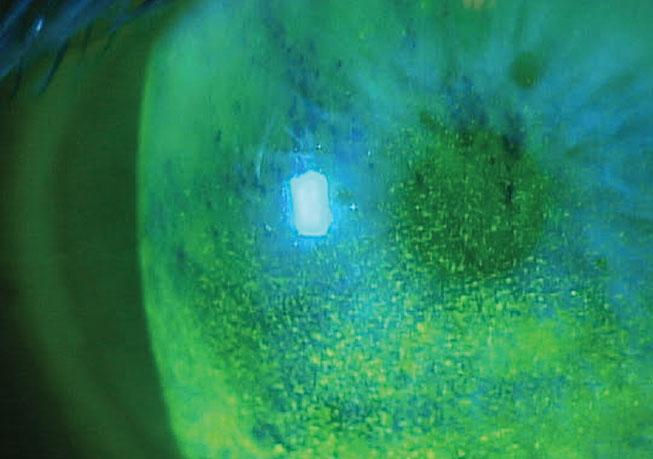 |
|
Benzalkonium chloride can cause a host of problems to the ocular surface; in this case, toxicity is present. Photo: Paul Karpecki, OD. Click image to enlarge. |
Sunscreens, topical steroids and various ointments and creams can cause negative ocular effects. Mainly, ones to look out for are topical acne and rosacea meds containing alpha hydroxy acid (glycolic acid), beta hydroxy acid (salicylic acid), retinoids and ivermectin.
Systemic drugs can equally contribute to ocular surface problems, with emphasis in the report placed on conditions of Stevens-Johnson syndrome and toxic epidermal necrolysis. Sao Paulo’s José Gomes, MD, lead author of this subcommittee report, brings attention to the fact that these conditions “develop after patients with genetic predispositions take these medications to treat a specific infection and develop an acute reaction that can evolve to chronic cicatricial changes in the ocular surface, compromising the vision of the patient.” But even simple cold meds, widely used in the population, can have deleterious effects, he notes.
Drug-induced dry eye, whether from topical or systemic compounds, should be treated by identifying the culprit and stopping administration if possible or switching to something else. Topically, this means using preservative-free or low-toxicity preservative drugs.4
Surgical procedures focused on or around the eyes—e.g., blepharoplasties, ptosis repair, canthoplasty, brow lifts—can cause various types of ocular surface damage. Botox injection is a first-line therapy for blepharospasm and hemifacial spasm but one study found it induced ptosis in 8.4% to 13.4% of cases, transient tearing in 5% to 10%, dry eye in 3% to 7.5%, photophobia in 2% and ectropion in 1%, the report states.4
Conversely, interventions such as punctal plugs and low-level light therapy seem to help with dry eye, the latter specifically improving meibomian gland dysfunction. Pinguecula and pterygium excisions both show some post-op improvement in dry eye symptoms, as does conjunctivochalasis.4
Using CO2 or Er-YAG lasers can result in conjunctival hyperemia, corneal ulceration and bullous keratopathy or ectropion. Refractive surgery’s potential to induce post-op dry eye is well known. One study of LASIK patients found dry eye persists in up to 94.8% at one day, 85.4% at seven days and 59.4% at 30 days. More worrying is LASIK-induced neuropathic epitheliopathy, which develops in up to 4% of patients, according to the report. PRK patients had dry eye up to six months post-op in 43% in one study. However, risk factors are known and screened for: pre-existing dry eye, female sex, Asian race, contact lens use and older age.
Other procedures that have resulted in dry eye are corneal crosslinking (with epi-on displaying better OSDI scores than epi-off), cataract removal and gamma knife radiosurgery for trigeminal neuralgia. With cataract surgery, dry eye improvement typically occurs by three months post-op, though may take up to six in diabetic patients.
Taking the multitude of procedures and medications into consideration, Dr. Gomes points to a few ways to prevent or reduce dry eye and other ocular surface problems. “Adverse events may be reduced by changing to a different class of topical medication, using corticosteroids, lubricating the eyes frequently and reducing exposure to preservatives.”
He also puts responsibility on practitioners to “stress that increasing the awareness of the potential risks, benefits and consequences will help patients to make the right decisions when considering when to undergo elective procedures and medications.”
Environment & Climate
We cannot easily change the environment we live in, but at least the knowledge of which factors are contributing to ocular surface health can aid in developing solutions.
The climate itself has many contributors to ocular surface status. Extreme high or low temperatures both in- and outdoors have been associated with dry eye, and temperature variations may be related to allergic conjunctivitis. Low humidity has been linked to greater ocular irritation, with one study showing improvement of symptoms with indoor humidity of 30% to 40%. Dry eye symptoms are exacerbated in low humidity environments like in deserts, airplane cabins and during dry seasons.
Wind speed is not well-documented in relation to ocular surface effects, but cases of corneal freezing and frostbite exist in ultra-marathon runners exposed to high wind speed and military freefall parachutists experiencing high wind, respectively. One suggestion for those living or working in cool, low humidity and windy environments is to wear silicone hydrogel contact lenses rather than hydrogels, due to the latter showing greater dry eye and visual disturbance symptoms. Dew point—the temperature at which air must be cooled to reach maximum water saturation—may serve as a protective factor against dry eye with higher levels.
High altitude is known for causing short-term effects of corneal thickening and long-term effects of dry eye and pterygium due to hyperbaric conditions, stronger ultraviolet (UV) radiation compounded by higher number of sunshine hours, low air pressure and dry and cold air. Often coinciding with high altitude, UV radiation is associated with conjunctival and eyelid malignancies and climatic droplet keratopathy; it primarily affects outdoor workers.
Recently, more extreme weather conditions like increased temperatures and precipitation have caused longer pollen seasons and higher indoor and outdoor mold spore concentrations, resulting in more allergic conjunctivitis and ocular allergic symptoms, potentially exacerbating dry eye as well.
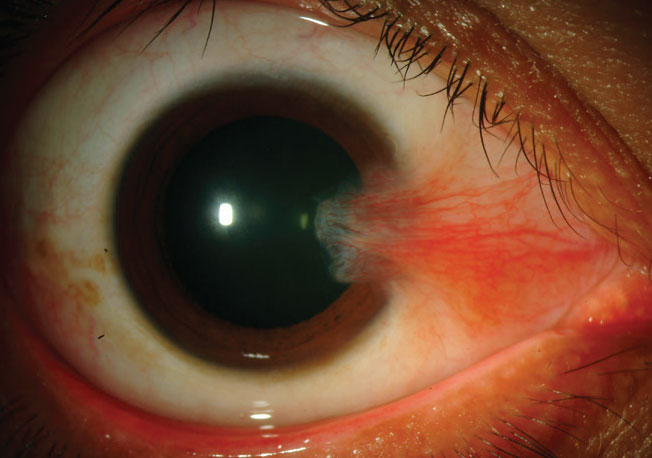 |
|
Pterygia are fairly common among those living in higher altitudes or closer to the equator. Photo: Christine Sindt, OD. Click image to enlarge. |
Pollution is another source of negative health effects. This subcommittee’s systematic review investigated whether specific chemical pollution compounds are related to dry eye incidence. They found it to be greater with exposure to air pollutants of nitrogen dioxide (NO2) and carbon monoxide (CO), as well as with soil pollution from chromium. NO2 has been associated with greater ocular irritation, lower tear break-up time and increased meibomian gland dysfunction, while CO is likely associated with increased dry eye symptoms. However, there was no increased dry eye prevalence with particulate matter. Dry eye disease and conjunctivitis have also been correlated with both indoor and outdoor pollution.
Two conditions of note are “sick building” and “sick house” syndromes, which display ocular symptoms. Similar in nature, both describe a scenario where the occupant experiences acute health effects directly linked to spending time in a work building or the home, with symptoms subsiding once away from the premises. Symptoms can range from chemosensory changes to skin symptoms, but as it relates to the eye, the most common side effects are tired or strained eyes, dryness, itchiness, irritation and watering. Since these symptoms may also be signs of ocular conditions of dry eye disease, refractive error or conjunctivitis, it’s hard to parse whether symptoms are always due to sick building syndrome or are indicative of a building-related illness.5
Either way, patients should be made aware of the possibility of their work and/or living space making their ocular symptoms worse. The syndromes are likely caused by many interrelated factors. This can be seen through the association of sick building syndrome symptoms and female sex, asthma or parental asthma history, pollen or pet allergy, humidity, dampness, office crowding, lack of office cleanliness and more.5
The report devotes some attention to the common finding of pterygia, which has a fairly high prevalence of 10% to 12%, with higher incidence closer to the equator. This is unsurprising as it is linked to prolonged sunlight exposure, higher altitude and outdoor exposure.
Covered last in this section is the range of ocular surface injuries that can occur. Chemical injuries can occur from occupational exposure, especially from construction and agricultural work. Household exposure can result from cleaning agents and hydrogen peroxide. Thermal injuries happen from direct flames, scalding liquid, burning items, curling irons or fireworks. Many of these are risks that people are exposed to daily, so informing patients of the risks of these agents or exposures may help them to be cautious, especially toward their eyes.
Nutrition
Unlike some other categories covered, this subcommittee report found the scientific grounding to be lacking overall in solid evidence across many facets of nutrition, since the literature is sparse. However, there is still enough to look at many aspects and likely associations, or lack thereof, with dry eye.
The most solid evidence exists for dietary components of omega-3 and omega-6 polyunsaturated fatty acids. Omega-6 does not benefit the ocular surface but omega-3 does. Greater omega-6 consumption correlated with a 2.5x higher risk of dry eye, while a 30% reduction in risk was observed with each gram of omega-3 consumed each day. This may be due to omega-6 being more pro-inflammatory while omega-3 exhibits greater anti-inflammatory properties. As such, the ideal ratio of consumption of omega-6 to omega-3 is less than 4:1. Despite this, Maria Markoulli, PhD, MOptom, the primary author of this subcommittee and a member of UNSW Sydney’s School of Optometry and Vision Science, still points out that “what we don’t yet know is what the optimal dietary intake needs to be to prevent and manage dry eye disease.”
Macronutrients most important in maintaining ocular surface health include vitamins A2,3, B12, C and D. Vitamin A deficiency can cause reduced or absent goblet cells and corneal punctate keratopathy. Long-term, this deficiency can lead to corneal perforation and other effects. Vitamin B12 deficiency was associated with a 1.5x increased risk of having dry eye, the report found. Vitamin C is present in human tears and serves as an antioxidant defense as well as helping heal the cornea following injury, suggesting it could play a role in dry eye if insufficient.
There is much evidence of vitamin D deficiency impacting dry eye, including its ties to the pathogenesis of the disease. Selenium and lactoferrin may play a role in maintaining the ocular surface, as decreased levels of selenium in tears and decreased lactoferrin levels were linked with dry eye.6
Although hydration offers plenty of benefits for many organs and organ systems, it does not seem to be indicative of a protective factor against dry eye or other ocular surface outcomes with increased intake, the report found.
Endocrine-disrupting chemicals may be consumed through ingestion, leaching from food containers, and end up changing gut microbiome diversity. Blood concentration of mercury is linked to dry eye symptoms, with main consumption happening through contaminated fish. Alcohol is weakly associated with dry eye, but with no increased risk seen in heavy drinkers. Other dietary factors increasing the risk of dry eye are anorexia nervosa, food intolerance and food allergy. About 14% of those who possess some form of food allergy have allergic conjunctivitis and it puts patients at an almost 1.5x increased risk of dry eye development.6
As far as specific habits are concerned, only the Mediterranean diet is directly linked to the ocular surface by decreasing dry eye symptoms and risk of Sjögren’s syndrome, likely due to anti-inflammatory properties from olive oil and nuts.
 |
|
The Mediterranean diet has been shown to reduce risk of dry eye. Photo: Getty Images. Click image to enlarge. |
Dietary additives or supplementation of certain agents have been studied on their effects on the ocular surface. Caffeine may actually have a slight protective effect against dry eye, as might manuka honey, which decreased allergic symptoms with dietary birch pollen honey. Dr. Markoulli points to “oral ingestion of multiple Chinese herbs that have been reported effective against Graves’ ophthalmopathy and dry eye, and curcumin has been found to reduce oxidative stress, angiogenesis and inflammation.”
Vitamin A supplements reduce dry eye symptoms compared with no treatment or treatment of cyclosporine, according to the report. Vitamin B12 administration similarly improves dryness symptoms. Omega-3 is also effective as a supplement to decrease dry eye symptoms and signs, but optimal dosage, composition and duration of supplementation treatment still needs to be determined.
Nutrition extends beyond the food we eat, also comprising the gut microbiota. As Dr. Markoulli notes, “the gut microbiome plays an important role in the regulation of low-grade chronic inflammation and ecological shifts within the gut microbiome can induce imbalance or dysbiosis, which is associated with chronic disease.”
Specifically, dry eye is associated with severe gut dysbiosis and reduced microbiome diversity. Luckily, pre- and probiotics may help to improve dry eye symptoms. This approach to dry eye may be something to look out for in the future, as Dr. Markoulli mentions “the field of modulating the gut microbiome as an intervention to treat dry eye disease is relatively in its infancy.”
Diseases related to nutrition can also impact the ocular surface. Obesity, metabolic syndrome, diabetes, cardiovascular disorders, chronic kidney disease, inflammatory bowel disease and irritable bowel syndrome are all linked with abnormal ocular surface changes and increased dry eye prevalence, symptoms and/or signs. Ocular complications can result more often after bariatric surgery due to malabsorption. For some reason, however, hypertension is inversely linked with dry eye prevalence.
Societal Challenges
This section comprises factors that are out of one’s personal control and instead are what might be considered issues that need to be addressed by entire countries or cultures.
However, perhaps the most obvious factor beyond our control is our biology and the aging process. Dry eye is increasingly prevalent as age increases and the related condition of meibomian gland dysfunction also increases with age, potentially exacerbating or contributing to dry eye.7
Sex is a risk factor for dry eye, with female patients more at risk, as well as observed biological and physiological differences seen in structures of the cornea, conjunctiva, lacrimal glands, meibomian glands and tear film. This yields an almost twofold higher risk for women to develop dry eye compared with men. However, higher rates of asymptomatic meibomian gland dysfunction are seen in Caucasian males.7
Southeast Asians have 1.5x to 2.0x higher risk of having dry eye disease and meibomian gland dysfunction, the report points out. Heritability of dry eye seems to sit at about 30% for symptoms and 40% with a previous dry eye disease diagnosis. Signs of dry eye range from 25% to 80% in heritability.7
Also outside of many people’s capabilities are fixing issues of malnutrition or food insecurity, as without proper nutrition, the eye (as well as the rest of the body) cannot function optimally. Water, sanitation and housing are all factors associated with trachoma development and other waterborne diseases.
Fiona Stapleton, PhD, MCOptom, also a part of UNSW’s School of Optometry and Vision Science, specifies as this subcommittee’s head that “there’s also a very strong link between education and poverty, socioeconomic class and access to health services which will affect both the prevalence and severity of many ocular diseases.”
Remoteness, geography and seasonality can all impact the types of ocular surface diseases seen and the severity displayed. Along with this, the availability and affordability of services will vary by region. Ocular surface disease presence will be compounded by the cost of diagnostics and treatment, with options of national health services, insurance or out-of-pocket expense weighing on patients with how likely they will be able to afford treatment.
 |
|
Global pandemics not only expose vast populations to disease but also to numerous second-order effects, such as COVID’s mask-induced dry eye and greater reliance on digital screen time. Photo: Jakayla Toney/Unsplash. Click image to enlarge. |
Occupation affects how likely patients are to develop ocular surface diseases. Exposure, both short- and long-term, to corrosives and excess heat can cause acute or chronic ocular surface injury and complications. Cleaners, miners, construction workers, laboratory workers, food industry workers, agricultural workers, fire workers and mechanics should take caution with adequate protective eyewear to minimize their heightened risk of ocular surface burns. Working night shifts is a risk factor for meibomian gland dysfunction, tear film instability and worsening dry eye symptoms. Prison populations experience poorer health outcomes from lack of services, nutrition limitations and lack of awareness of disease. Conjunctivitis, xerophthalmia and pterygia are all conditions overrepresented in this population.
Interestingly, higher education may be associated with dry eye, due to increased screen use in white collar jobs. Related to this, socioeconomic status is well-regarded as a contributor to ocular surface disease more generally, with lower status experiencing greater burden. Accessibility to helpful resources and cost still remain issues to many.7
With COVID having disrupted lives since 2019, this subcommittee wanted to clarify how the pandemic has impacted ocular surface diseases. First, they looked at screen time use, implementation of online classes and digital device use during the pandemic. Eye symptoms were found to worsen with exposure to screen time and online learning as a consequence of the pandemic, with no ocular symptoms of dry eye improving with exposure. As well, there was worsening or no change associated with signs and symptoms of dry eye when exposed to the virus.
Strategies to mitigate virus spread, like face masks, were also analyzed. Mask wear was found to both induce and exacerbate dry eye signs and symptoms, as well as cause contact lens intolerance and more cases of chalazia. However, allergic ocular symptoms decreased with mask use, probably from a barrier of the nasal passage to exposed airborne allergens.
Dr. Stapleton explains that since remote, hybrid and flexible work are likely to continue, it is reasonable to assume that frequency and severity of ocular surface diseases will continue along with this. As such, practitioners, she believes, “can be proactive with their patients to mitigate these effects.” She also puts into perspective how these larger, societal factors are at play with the ocular surface. “Focusing on social determinants and their interactions, rather than just the cause of the disease, encourages the development of more comprehensive, holistic solutions and a ‘whole of society’ approach to reducing disease morbidity. It encourages practitioners to have a broader discussion with their patients about lifestyle and social determinants.”
Personal & Lifestyle Challenges
In the last of the reports, more patient-specific issues are addressed, covering mental health, physical health and lifestyle choices. Although these factors may not initially seem relevant to the ocular surface, there are some surprising associations.
There has been an increasing awareness of how mental health disorders affect the rest of the body. This report covers mainly the conditions of depression, anxiety and generalized stress.
First discussed was depression. An alarming 29% of individuals with dry eye disease also had depression, as one meta-analysis concluded. A different one was able to determine that dry eye disease patients had a higher frequency of depression. Interestingly, dry eye symptom scores were associated with depression severity scores, but data is much weaker when looking at dry eye signs. Of note, both depression and antidepressant medications may be linked to dry eye with a biologic basis, as evidenced by SSRI antidepressants having been linked with ocular surface changes, too.
Anxiety disorders saw a similar trend as with depression. Several studies reported a link between anxiety and a dry eye diagnosis. A PTSD diagnosis increased odds of a dry eye diagnosis twofold, and anxiolytic use is a strong predictor of dry eye diagnosis as well. Similar to depression, there is an association between dry eye symptoms and anxiety, but not signs. However, much of what’s been found was done on veterans, so the general population may exhibit differences.
Relieving dry eye symptoms may help with anxiety, as one study found after treating dry eye patients with various methods for three to six months. While dry eye has not been studied specifically with mental health treatments, therapy has helped with other chronic pain conditions.8
The effects of stress on the eye are apparent, as worse self-perceived health status and more psychological stress were both linked with increased susceptibility to aqueous deficient and evaporative dry eye and its symptoms.
When sleep is interrupted, it can cause serious issues to the body. Dry eye patients have poorer sleep quality, spend less time asleep and experience more sleep disturbances. Sleep-related factors like excessive sleepiness, insomnia, high sleep apnea risk and receiving less than five hours of sleep are associated with dry eye symptoms, showing a clear connection between the need for proper sleep and maintenance and repair of the body. Primary Sjögren’s similarly exhibits increased sleep disturbances, more night awakenings and obstructive sleep apnea presence. However, being able to get adequate rest may revert some observed ocular surface changes, as this was described in animal models.
Obesity may put patients at risk of worsening dry eye, as it has been connected to evaporative dry eye, tear film instability and meibomian gland abnormalities in function and architecture. Unfortunately, treatment for often comorbid obstructive sleep apnea in obese patients has also been linked to dry eye diagnosis and has shown to cause greater ocular irritation upon initiation, with CPAP device use having been reported to induce ocular manifestations in as high as 50% to 70% of patients.
This subcommittee undertook the task of reviewing chronic pain conditions and whether they are contributive risk factors to dry eye disease for their systematic review. One study cited as much as 17% of patients have at least one chronic pain condition. They reviewed many conditions, but found that some were indeed linked with an increased risk of dry eye. These included migraine with a 1.61x increased risk, fibromyalgia with a 1.91x increased risk, irritable bowel syndrome with a 2.16x increased risk and back pain with a 1.60x increased risk. All these observations suggest a comorbidity between chronic pain conditions and dry eye, but mostly with symptoms.
 |
|
Vaping is just one lifestyle choice patients can make that has direct negative consequences on their ocular health. Photo: Getty Images. Click image to enlarge. |
Drugs can affect the ocular surface differently, depending on the type. Kelly Nichols, OD, of University of Alabama Birmingham’s School of Optometry and one of the authors of this report, points to the adverse effects of smoking on ocular surface health.
“Surprisingly, there’s not a lot of good data to support that, even though everyone would suggest that probably you shouldn’t smoke because it could impact your eyes. It doesn’t mean the association isn’t there, just that the literature gives us less guidance than we may want.” She advises ODs to look a little more carefully at the tear film, and tear film stability in particular, in a patient who’s a smoker. “It could potentially have more impact on the ocular surface, whereas symptoms in that individual may not play as significant a role, or at least they’re not telling you that.”
Vape users experienced greater dry eye disease symptoms and signs compared to non-smokers, the report found, and cannabis use long-term may decrease corneal endothelial density. Alcohol intake is a contributory factor to dry eye, but this may be part of a wider issue of poor nutrition. Caffeine, however, actually shows evidence of increasing tear meniscus height and Schirmer test values two hours after intake, suggesting it may produce better tear parameters.
Dr. Nichols offers some key takeaways to carry into your practice from the findings of this section. “Many patients are either taking anti-anxiety or antidepressant medications. These therapies, and the underlying conditions, also overlap with sleep disorders, and all of these have potential consequences for the ocular surface. So, asking them about their sleep and the comfort of their eyes might be a really important thing to do. Creating conversations around some of these less obvious connections to dry eye might be a positive outcome of the report.”
Takeaways & Concluding Remarks
One of the recurring themes throughout all sections of this massive report is that doctors may need to change some of the recommendations they’re giving to patients.
“We have to be very careful that we’re not just doing something for the sake of it, that we actually have the evidence behind it to support doing that, and we don’t have patients investing money in things that might not work,” Dr. Craig cautions.
This might be most evident when reviewing the subcommittees’ findings on blue-light blocking lenses, hydration or smoking and their effects on the ocular surface.
“It’s a really important area to find out more information, but we need better quality studies. We don’t have enough evidence yet. The report isn’t saying blue light doesn’t elevate certain risks. We need to use that information to help drive future research until we do have enough evidence to decide whether things cause a problem.”
Dr. Craig is excited by the prospects for change, and thinks that education on these topics will be reciprocally exchanged by clinicians and their patients in the wake of the TFOS Lifestyle Report, pointing out that patients now more than ever are engaging themselves in becoming informed about their health.
She hopes that these sort of integral conversations between patients and doctors will “help drive better standards in industry and things like cosmetics. If there’s resistance from the professions and from the public, then they may be forced to change some of the components within their cosmetics if they’re no longer accepted. If it becomes recognized that they cause problems, I think we should see changes happening there.”
Cosmetics is one of the most apparent industries in which regulations and manufacturing processes need to be changed to reflect what is currently known about its effects on health, but this is not the only area that needs change.
Through the combined efforts of all the subcommittee reports, we now know more than ever about a wide range of factors that contribute to ocular surface health. Heed the advice and tips given by the researchers of this report, continue to adapt your recommendations as the literature evolves, and you’ll be in the best position to help your patients.
 |
| Click image to enlarge. |
1. Jones L, Efron N, Bandamwar K, et al. TFOS Lifestyle: impact of contact lenses on the ocular surface. Ocul Surf. 2023;29:175-219. 2. Sullivan DA, da Costa AX, Del Duca E, et al. TFOS Lifestyle: impact of cosmetics on the ocular surface. Ocul Surf. 2023;29:77-130. 3. Wolffsohn JS, Lingham G, Downie LE, et al. TFOS Lifestyle: impact of the digital environment on the ocular surface. Ocul Surf. 2023;28:213-52. 4. Gomes JAP, Azar DT, Baudouin C, et al. TFOS Lifestyle: impact of elective medications and procedures on the ocular surface. Ocul Surf. 2023;29:331-85. 5. Alves M, Asbell P, Dogru M, et al. TFOS Lifestyle Report: impact of environmental conditions on the ocular surface. Ocul Surf. 2023;29:1-52. 6. Markoulli M, Ahmad S, Arcot J, et al. TFOS Lifestyle: impact of nutrition on the ocular surface. Ocul Surf. 2023;29:226-71. 7. Stapleton F, Abad JC, Barabino S, et al. TFOS Lifestyle: impact of societal challenges on the ocular surface. Ocul Surf. 2023;28:165-99. 8. Galor A, Britten-Jones AC, Feng Y, et al. TFOS Lifestyle: impact of lifestyle challenges on the ocular surface. Ocul Surf. 2023;28:262-303. |

