Dry eye disease is one of the most common clinical entities optometrists encounter today. However, our discipline has yet to reach a consensus on the rationale for diagnosis and treatment. In addition, it is well established that the signs and symptoms of this condition are not congruent, and missed or false diagnosis remains a critical hurdle in the long-term management of this chronic disease.1
Dry eye disease is a complex dysfunction of the ocular surface and is characterized by sometimes-painful symptoms, increased tear evaporation, inflammation and reduced tear production.2 Research suggests the activation of T-cell mediated pro-inflammatory immune responses lead to cytokine release, inflammation and a hyperosmolar state.3 For this reason, most therapies target the disease’s inflammatory nature.
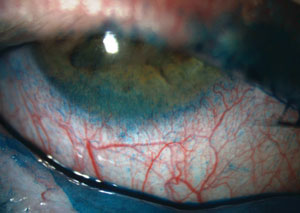 | |
| Severe staining with lissamine green on the conjunctiva and, particularly, on the inferior cornea. |
The market, like nature, abhors a vacuum and with so few available dry eye treatments (and an ever-growing number of dry eye patients), a vacuum certainly exists. The pharmaceutical industry is on the cusp of offering several new formulations to the market.
This article provides a look at what treatments are in development, possibly on their way to our offices and how they work.
What’s the Hold Up?
Before reviewing drugs not yet available, consider that, to date, the only FDA-approved medication for the management of dry eye is Restasis (topical cyclosporine 0.05%, Allergan). Restasis was the first agent to target the T-cell related inflammatory pathways in dry eye disease. In particular, it is approved for the reduction in inflammation of the lacrimal gland. That was approved 13 years ago. You may be wondering ‘what’s the hold up?’
On a basic level, the disease’s complexity is to blame. Based on previous FDA approvals and subsequent denials of new drug applications (NDAs), it appears that a candidate dry eye drug in pivotal trials should demonstrate statistical significance in the reduction of both signs and symptoms of dry eye—no small task. Are we making any headway? Let’s take a look at the efforts to find the next blockbuster drug for dry eye disease.
1. Cyclosporine
While the current formulation of Restasis dominates the market, the next generation of cyclosporine could be just around the corner. Allergan has enjoyed an exclusive position in the dry eye pharmaceutical area and, indeed, is working on its own update in Restasis X. In the future it may have to compete with Ikervis (cyclosporin A 0.1%, Santen), which was recently approved in Europe. In addition to a higher concentration, Ikervis employs new delivery methods, such as new vehicle technology that may make cyclosporine more tolerable and perhaps more efficacious. The proof will be in the clinical trials currently underway.
Lifitegrast Data Undergoing FDA Review |
Ikervis is approved throughout Europe for the treatment of severe keratitis in adults with dry eye that has not improved, despite treatment with tear substitutes. It appears, via clinicaltrials.gov, that Restasis X (also a 1% concentration) is under review by European regulators too.
2. Lifitegrast
When considering mechanisms of action for potential new drugs in the dry eye arena, most efforts have been devoted to inflammation, tear production, tear film movement and tear chemistry; specifically, lipid layer chemistry. Additionally, drug delivery platforms remain a viable development goal in terms of increased efficacy, convenience and compliance for patients.
Lifitegrast is an agent that its parent company, Shire, hopes to move expediently toward the commercialization process. The agent itself mimics lymphocyte function antigen-1 (LFA-1), thereby preventing activation of intercellular adhesion molecule-1 (ICAM-1) which is expressed on the inflamed epithelial cell surface. Activation and migration of free lymphocytes to the ocular surface are key steps in the chronic inflammatory process leading to dry eye disease. This process is influenced and initiated by the binding of the T-cell integrin LFA-1 to ICAM-1. The drug influences the activation and homing of activated T-cells or cytokines. Lifitegrast acts as an ICAM-1 decoy and subsequently prevents binding of LFA-1 to ICAM-1, breaking the cycle of T-cell mediated inflammatory response on the ocular surface.
3. EBI-005
Eleven Biotherapeutics has developed an agent it is currently calling EBI-005 that targets the inflammatory pathway in a much different way, via the IL-1 system.
IL-1 is a cardinal mediator of inflammatory responses and likely plays a key role in the modulation of signs and symptoms of dry eye disease. There are two IL-1 cytokines, IL-1 alpha and IL-1 beta, the latter of which regulates immune function and T-helper differentiation involved in the inflammatory cascade. IL-1 also mediates pain from the corneal nerve plexus implicated in the symptoms of dry eye disease.
EBI-005 is currently in Phase II clinical trials for the treatment of dry eye. These trials will look to demonstrate whether EBI-005 can block both inflammation and pain associated with dry eye.
4. Anakinra
Another IL-1 antagonist significantly reduced symptoms and corneal epitheliopathy in dry eye patients, according to research published in 2013.4 That study looked at 75 patients using Kineret (anakinra, Amgen) and measured dry eye-related symptoms by using the Ocular Surface Disease Index, tear film break-up time and meibomian gland secretion quality. After 12 weeks, the study showed significant reductions in dry eye symptoms of 30% in patients who received topical anakinra (2.5%) and 35% in patients who received a higher dose anakinra (5%).4
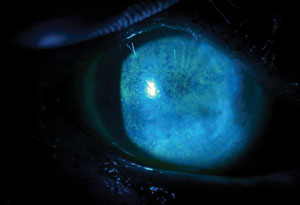 | |
| Fluorescein staining pattern of a patient with severe punctate epithelial keratopathy secondary to dry eye, associated with Sjögren’s syndrome and exposure keratopathy. |
The study showed no reports of serious adverse reactions attributable to the therapy.6
5. MIM-D3
MIM-D3 (Mimitogen Pharmaceuticals) is a topical drug in the dry eye pipeline that is different in its approach to dry eye therapy. It is a molecule that has multiple activities, including survival and differentiation of neuronal cells, stimulation of mucus secretion and participation in the repair of corneal epithelial cell damage. It partially mimics nerve growth factor, which research shows may improve the clinical outcome of neurotrophic keratitis and corneal ulcers.7,8
The molecule that nerve growth factors work through is tyrosine kinase (TrkA) which is found in human conjunctival epithelial cells. MIM-D3 also serves to mimic TrkA. Phase II clinical trials have concluded and a multicenter Phase III clinical study for the treatment of dry eye syndrome is currently underway.
6. Rebagen
Rebagen (rebamipide, Otsuka Pharmaceuticals) was originally researched for the treatment of gastric ulcers in rats and later marketed as a tablet for gastric ulcer therapy.
However, it was later found that the drug also has the ability to reduce inflammation and promote epithelial mucin secretion. Research shows its ability to increase the secretion of corneal and conjunctival mucins and to increase the number of goblet cells in rabbits.9 Currently, it’s only available for dry eye treatment in Japan.
7. Tofacitinib
Tofacitinib (Pfizer), a topical ophthalmic Janus kinase (JAK), may also act as an immunomodulator in dry eye patients. This medication essentially blocks signaling that leads to inflammation. In a clinical trial that looked at 327 patients for eight weeks, doctors observed clinically significant improvements to signs and symptoms of dry eye.10
Nanotechnologies and Other Novel Delivery Methods Eyegate is investigating another drug delivery system to deliver dexamethasone via iontophoresis. This technology works by applying a small electrical current to the ocular surface to increase drug concentration and enhance conjunctival and scleral absorption. Other, more familiar technologies include punctal plug drug-eluting devices containing agents ranging from dexamethasone to cyclosporine. |
One study, albeit a small one with only 82 patients, showed a promising reduction of conjunctival cell surface HLA-DR expression and tear levels of proinflammatory cytokines and inflammation markers after eight weeks of treatment.11
A 2014 animal study showed tofacitinib treatment decreased corneal infiltration of the CD45+, Gr-1+ and CD11b+ cells on days one and three of treatment.12
Tofacitinib and other kinase inhibitors are known for their ability to treat rheumatoid arthritis.13
Nicox acquired several kinase inhibitors last year in a deal with Aciex Therapeutics. That deal included a small molecule dual Syk/JAK inhibitor for potential topical treatments, a drug being developed for allergic conjunctivitis (AC-170), and another for postoperative inflammation and pain (AC-155), according to a press release.
8. EGP-437
EyeGate Pharmaceuticals’ take on dexamethasone phosphate formulated for ocular iontophoresis is useful as a tool against both uveitis and dry eye, according to the company’s website. But does the science pan out? According to a 2011 study published in Clinical Ophthalmology, EGP-437 can demonstrate statistically and clinically significant improvements in both signs and symptoms of dry eye in a controlled adverse environment model.14 The randomized clinical trial looked at 103 patients. Unspecified treatment-emergent adverse events were experienced by 87% of patients and were consistent across all treatment groups. Most, however, were mild and none were severe, according to the study.
9. RGN-259
Perhaps the most recent addition to this list, RegeneRx’s thymosin β4 0.1% eye drops, RGN-259, was the topic of a study published in the May 2015 issue of Cornea. That study showed the results of a Phase II randomized trial in which the drug demonstrably improved both signs and symptoms of dry eye.15
Researchers have already seen that thymosin β4 upregulates the expression of laminin-5, a component of the basement membrane region of the skin, cornea, conjunctiva and other tissues.15 These and other biological activities of thymosin β4 are important to corneal repair, according to a 2004 study.16
The results of the 2015 study show significant improvement in tear film break-up time at 28 days after the last treatment with RGN-259. Tear production was also shown to significantly increase at various points throughout treatment-—at day 7 (P = 0.0001) and day 21 (P = 0.0449)-—and continued improvement was observed at a 28-day follow up. None of the patients studied reported any adverse events.15
10. KPI-121
Kala Pharmaceuticals recently announced results from a Phase II trial of KPI-121, a nanoparticle formulation of loteprednol etabonate that uses the company’s mucus-penetrating particle technology. Loteprednol etabonate is an anti-inflammatory corticoid known as the active ingredient in Lotemax (loteprednol, Bausch + Lomb). But that mucus-penetrating particle technology is the star of the show. Mucus-penetrating particles are a variation of nanotechnology. They allow diffusion of small particle size medications to the mucin layer.
As the demographic suffering from dry eye skyrockets over the next decade, new treatments will be crucial for managing this burdensome disease. Clearly, there is a gap between today’s needs and effective, broad-based treatments and the gap can be expected to widen without new therapeutic options.
Dr. Gaddie is the owner and director at Gaddie Eye Center in Louisville, Ky.1. Nichols K, Nichols J, Mitchell G. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004 Nov;23(8):762-70.
2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92.
3. Stevenson W, Chauhan S, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012 Jan;130(1):90–100.
4. Sheppard J, Torkildsen G, Lonsdale J, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014 Feb;121(2):475-83.
5. Karpecki P, Tauber J, Raychaudhuri A, Semba C. Lifitegrast ophthalmic solution 5.0% vs. placebo for treatment of dry eye disease: results of a Phase III, randomized, controlled trial (Opus-2). Paper presented at American Academy of Optometry Meeting 2014, November 13; Denver.
6. Amparo F, Dastjerdi M, Okanobo A, Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. 2013 Jun;131(6):715-23.
7. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–9.
8. Lambiase A, Rama P, Bonini S, et al. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med 1998;338:1174-80.
9. Kashima T, Itakura H, Akiyama H, Kishi S. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: a critical appraisal. Clin Ophthalmol. 2014;8:1003–10.
10. Liew S, Nichols K, Klamerus K, et al. Tofacitinib (CP-690,550), a janus kinase inhibitor for dry eye disease: results from a Phase 1/2 trial. Ophthalmology. 2012;119(7):e43-e50.
11. Huang J, Yafawi R, Zhang M. Immunomodulatory effect of the topical ophthalmic Janus kinase inhibitor tofacitinib (CP-690,550) in patients with dry eye disease. Ophthalmology. 2012 Jul;119(7):e43-50.
12. Stevenson W, Sadrai Z, Hua J, et al. Effects of topical Janus kinase inhibition on ocular surface inflammation and immunity. Cornea. 2014 Feb;33(2):177-83.
13. Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012 Aug 9;367(6):495-507.
14. Patane M, Cohen A, From S. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol. 2011;5:633-43.
15. Sosne G, Dunn S, Steven P, Chaesik K. Thymosin ß4 significantly improves signs and symptoms of severe dry eye in a Phase II randomized trial. Cornea. 2015 May;34:491–6.
16. Sosne G, Xu L, Prach L, et al. Thymosin ß4 stimulates laminin-5 production independent of TGF-beta. Exp Cell Res. 2004;293:175–183.

