 |
When you’ve been in practice for many years and the number and variety of glaucoma scenarios you’ve encountered is rather large, you learn to manage these patients the best you can with the information that is available to you. For example, some patients physically are unable to perform visual field testing and OCT imaging; some cannot be examined with a slit lamp. Still others have media opacification that prohibits adequate visualization of the posterior pole. So, how do you handle them? This recent scenario is a perfect example of maximizing use of our technology to gather as much information as is possible.
Case
In early February of this year, a 92-year-old Caucasian woman presented to the office to establish care with me. She had been a lifelong resident of the area and was seen by a general ophthalmologist for many years before they retired. That practice was absorbed by a different group and she received care from this facility for the past few years. However, she grew disillusioned with the lack of personalized care, thus the change to my office.
She is a pleasant and spry 92-year-old who carries with her the following ophthalmic diagnoses: aphakia, severe macular degeneration, advanced glaucoma and low vision.
Her current ophthalmic medications included dorzolamide BID OU, 0.5% timolol BID OD and Rhopressa (netarsudil ophthalmic solution 0.02%, Alcon) HS OU. Systemic medications being taken included amlodipine QD, an unknown statin, Avapro (irbesartan, Sanofi), levothyroxine and vitamin D supplementation; she reported no known allergies to medications. Her entering visual acuities were 20/60 OD and hand motion at five feet OS. A refraction was not performed, as the aim of this initial visit was not refractive in nature. There was surgical distortion of both pupils and ipsilateral pupillary reactions were relatively normal. Extraocular movements were full in all positions of gaze. Applanation tensions were 14mm Hg OU at 10:20 AM. Pachymetry readings were 587µm OD and 585µm OS.
A slit lamp exam of the anterior segments revealed sectoral surgical iridectomies OU, vortex epitheliopathy OU, guttatae and mild striate keratopathy OU and a papilloma on the left upper lid nasally. Anterior chambers were deep and quiet OU with no cells, flare nor vitreous present bilaterally. The patient was aphakic OU.
Through dilated pupils, the vitreous was relatively intact without prolapse. The cup-to-disc ratio was estimated in vivo to be about 0.55x0.55 OD and 0.55x0.75 OS. The remaining neuroretinal rims were slightly pale bilaterally and there was clear erosion of the inferotemporal rim OS. There was significant peripapillary atrophy OU.
Both maculae were consistent with geographic atrophy (GA), with the left much worse than the right. Fortunately, the right foveal avascular zone was minimally affected by the GA. However, the GA in the left eye was extensive and coalesced with the peripapillary atrophy. The retinal vasculature was mildly attenuated and the peripheral retinal evaluation was remarkable for scattered areas of pavingstone degeneration.
Given the decreased visual acuity secondary to the GA as well as time limitations, a visual field was not performed at this visit, but OCT images were obtained bilaterally.
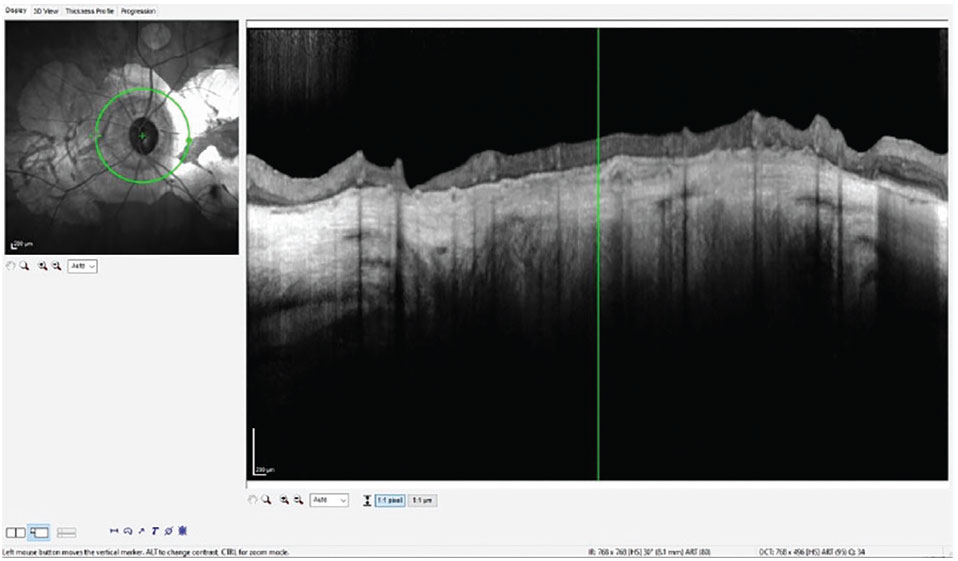
My glaucoma protocol in-office for OCT imaging involves using the GMPE software on the Heidelberg Spectralis, which offers many advantages, especially in cases such as this. Figure 1 is a typical OCT scan of the perioptic retinal nerve fiber layer (RNFL); in this case, of the patient’s left eye. Note the peripapillary atrophy (PPA) and the geographic atrophy affecting the entire area measured in this single circle scan. Notice also the paucity of perioptic neurosensory retinal tissue with loss of retinal integrity. This combination of GA and PPA in the perioptic area negates accurate interpretation of RNFL thickness, one of the fundamental markers of glaucomatous damage. In essence, progression of the glaucoma would not be able to be seen in this type of scan moving forward.
 |
|
Fig. 1. Note the complete undermining of the RNFL measurements by the PPA, thereby complicating interpretation of this parameter related to glaucoma. Click image to enlarge. |
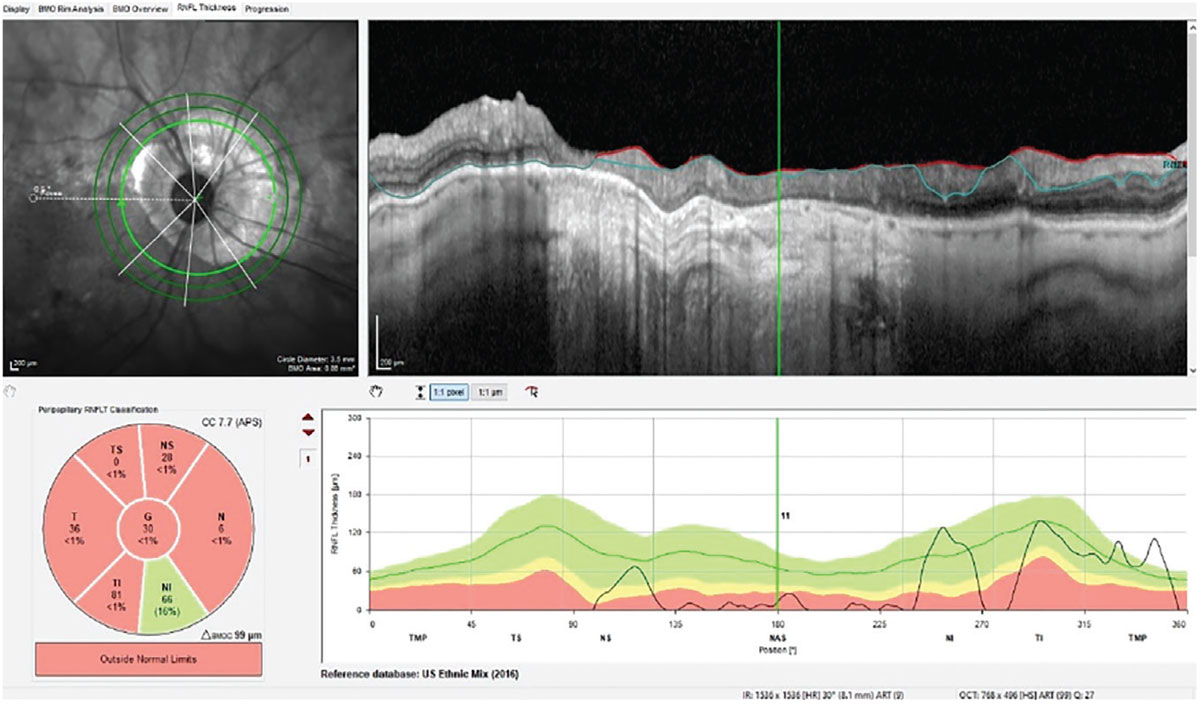
Compare and contrast Figure 1 with Figure 2. In Figure 2, we are looking at one of five GMPE software images available of the patient’s right eye. Specifically, this is at the 3.5mm RNFL circle scan. This particular scan is the innermost of three different diameter RNFL circle scans and is also affected by the PPA seen OD. Though the PPA OD is not as advanced as that of OS, it still affects interpretation of the RNFL thickness in this particular diameter scan. However, note that this is only one of three different diameter RNFL circle scans, with the other two being larger in diameter. In the next two larger 4.1mm and 4.7mm diameter scans, the entire temporal half of the RNFL scans are not influenced by the underlying PPA; this offers these circle scans the ability to detect glaucomatous progression in the temporal RNFL away from the optic nerve.
 |
|
Fig. 2. The 3.5mm RNFL circle scan of the right optic nerve using the GMPE software. Note the relatively unencumbered 4.1mm and 4.7mm diameter circle scans. Click image to enlarge. |
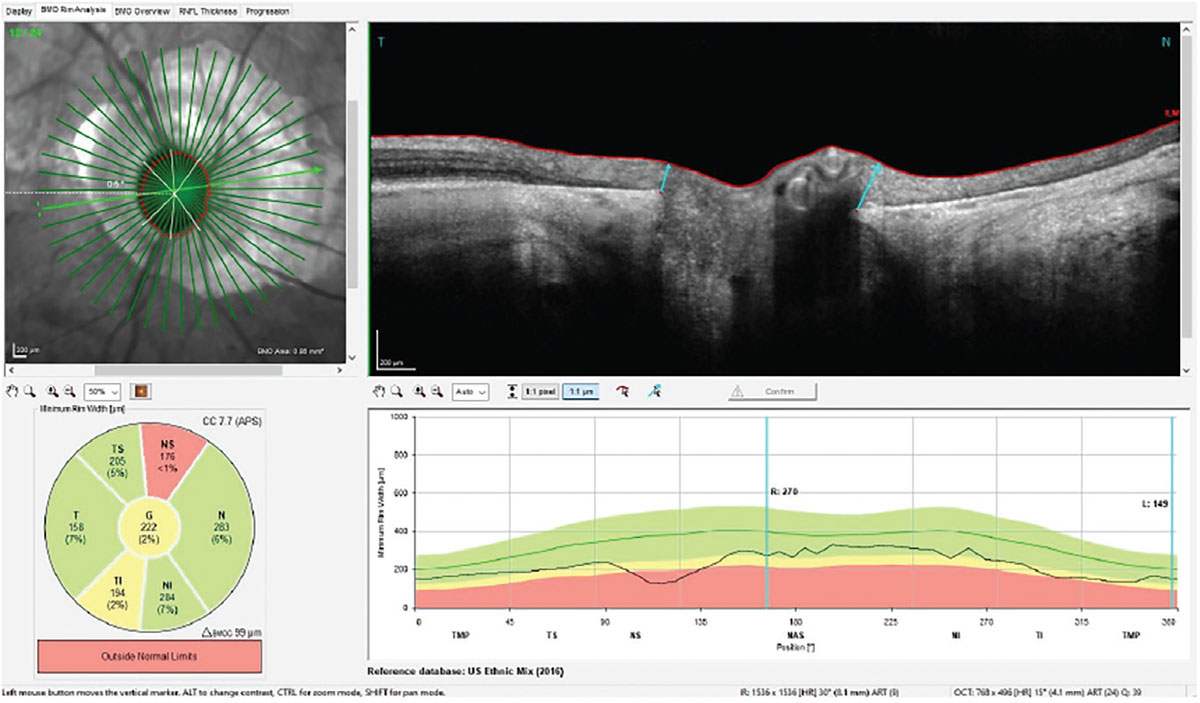
As has been mentioned in this column regularly, OCT analysis of the Bruch’s membrane opening minimum rim width (BMO-MRW) gives us details of the actual neuroretinal rim, seen in Figure 3. This shows individual locations of the radial scans through the optic nerve, each measuring the neuroretinal rim thickness. The individual scan highlighted is roughly a horizontal scan; note the relatively robust neuroretinal rim and clear edge of the PPA on the temporal side of the disc. Note also in the reference database plot the rather minimally-affected neuroretinal rim 360° in the right optic nerve, indicating moderate, not severe, glaucoma. This scan becomes the perfect one to use and examine in all follow-up visits, as the neuroretinal rim thickness measurements are not affected by the PPA.
 |
|
Fig. 3. The radial scans through the neuroretinal rim of the right eye measuring the BMO-MRW, which is completely unaffected by the PPA. Click image to enlarge. |
Discussion
If we were simply looking at one RNFL circle scan overlying a significant area of PPA, our ability to monitor the patient for progression would be severely limited. Having several different parameters of the posterior pole to evaluate in the context of glaucoma grants us the ability to find one or two parameters to use moving forward to monitor progression, such as the BMO-MRW demonstrated here.
Though field studies have not been performed on this patient yet, what strategy would be best used, given her decreased vision? When she is seen for her next visit, visual fields will be included via a VR headset field using the Olleyes VisuAll device. Since visual fields are obtained bilaterally, and given her better-seeing right eye, the right eye can be used as the fixating eye, hopefully giving us a bit more accurate visual field result (at least in the left eye) than if the left eye was unilaterally being tested and asked to fixate, which would certainly result in a poor-quality field result. Having the tools in your glaucoma toolbox helps tremendously to accurately manage your patients, independent of degree of glaucomatous damage present.
Dr. Fanelli is in private practice in North Carolina and is the founder and director of the Cape Fear Eye Institute in Wilmington, NC. He is chairman of the EyeSki Optometric Conference and the CE in Italy/Europe Conference. He is an adjunct faculty member of PCO, Western U and UAB School of Optometry. He is on advisory boards for Heidelberg Engineering and Glaukos.

