 Within medicine, the term giant usually suggests something ominous and foreboding. Giant retinal detachment and giant cell arteritis are just two examples that we encounter in eye care. In comparison to these sight-threatening maladies, giant papillary conjunctivitis (GPC) may seem like nothing more than an ophthalmic nuisance. But, for sufferers of this poorly understood chronic disorder, it is anything but a mere annoyance.
Within medicine, the term giant usually suggests something ominous and foreboding. Giant retinal detachment and giant cell arteritis are just two examples that we encounter in eye care. In comparison to these sight-threatening maladies, giant papillary conjunctivitis (GPC) may seem like nothing more than an ophthalmic nuisance. But, for sufferers of this poorly understood chronic disorder, it is anything but a mere annoyance.
Allergy-Related?
GPC has been loosely classified as a form of ocular allergy, mostly because it is known to involve mast cell degranulation.1,2 Patients with GPC are also likely to have a history of atopy (e.g., asthma, allergic rhinitis or hay fever).2,3 However, this condition differs from the more common clinical presentations of seasonal and perennial allergic conjunctivitis in several ways.
First, GPC has a greater eosinophil and basophil response than other acute forms of allergic conjunctivitis. In addition, tear histamine levels are comparatively low.1,4 From a pathophysiological perspective, GPC does not appear to be triggered by a foreign antigen like typical allergic reactions; rather, it is believed to occur due to a combination of traumatic and autoimmune elements.5 The chronic mechanical hammering on the tarsal surface by a contact lens or other object, such as an ocular prosthesis, combined with proteinacious surface deposits on these substrates has been hypothesized to induce a cell-mediated response, which results in papillary hypertrophy and other elements of surface inflammation.5
Patients with GPC present with ocular irritation or itching, increased mucus accumulation and variable palpebral conjunctival redness. The condition is associated with an object interacting with the ocular surface, such as an exposed suture, extruded scleral buckle, cyanoacrylate glue or ocular prosthesis.1-6
Most commonly, however, it is seen with contact lens wear, so practitioners sometimes refer to this condition as contact lens papillary conjunctivitis (CLPC). These patients generally report symptoms of contact lens discomfort and intolerance, including increased movement of the lenses, as well as surface coating and build-up.
Examination of these patients reveals large papillae along the upper tarsal conjunctiva; these papillae tend to be between 0.3mm and 1.0mm in size and are typically uniform in both shape and configuration.6 Additionally, tarsal conjunctival hyperemia and thickening may be observed. A stringy mucus discharge is another common finding.6
CLPC has been noted to occur with all types of contact lenses, though it is most common with HEMA-based hydrogels.1,6,7 Patients who wear soft lenses seem to develop this condition sooner, and are more symptomatic, than patients who wear gas-permeable or PMMA lenses. Silicone hydrogel lenses have also been implicated in the development of CLPC; however, the presentation in these patients may be somewhat abnormal. Research has shown that silicone hydrogels are more likely to induce an atypical, localized form of CLPC, in which the papillae are confined to just one or two areas of the tarsal conjunctiva near the lid margin.8,9
No matter what you call this condition, for the patient, it can be a constant source of irritation. Beyond the discomfort, CLPC may disrupt the patients vision due to mucus accumulation and excessive contact lens movement. Patients with CLPC are also often symptomatic when they remove their contact lenses because the papillae and ocular surface inflammation can have a direct impact on the cornea without the lens in place to serve as a bandage.
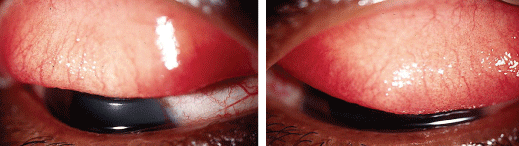
A patient presented with contact lens papillary conjunctivitis O.D. (left) and O.S. (right), complaining of lens-related discomfort.
Treat with Care
Identifying papillary conjunctivitis is usually straightforward and is based upon the characteristic constellation of signs and symptoms; however, management of this condition can be quite challenging. Virtually all sources agree that the initial treatment of this condition involves removing the inflammatory stimulus.1,2,5-9 In cases involving contact lenses, this typically means suspending lens wear until the condition resolves. Often, these individuals are either unwilling or unable to comply, and the practitioner must rely on pharmaceutical agents to hasten resolution.
Perhaps the oldest and most well-known therapeutic agents used in this capacity are the mast-cell stabilizers. Cromolyn sodium 4% was accepted as the gold standard in managing GPC throughout the 1980s and early 1990s.10-12 Since that time, newer mast-cell stabilizers, such as nedocromil, lodoxamide and pemirolast, have been used.
These agents prevent histamine release from mast cells, which makes them effective in the prophylactic management of Type I (immediate) hypersensitivity reactions. Unfortunately, these drugs can have a delayed effect of up to 10 days, can require q.i.d. dosing, and can have unpleasant side effects, including transient stinging and conjunctival injection.13
Additionally, GPC involves not only a Type I, but also a Type IV (delayed) hypersensitivity response that implicates T-cells rather than mast cells. So, a mast cell-stabilizing agent may prove only partially beneficial in this capacity.2
With the advent of combination antihistamine/mast-cell stabilizers, such as olopatadine, azelastine and epinastine, many practitioners have abandoned the pure mast-cell stabilizers in the treatment of CLPC and ocular allergies in general. The advantages of the combination agents include more rapid and complete symptomatic relief, less frequent dosing (once or twice daily) and greater comfort upon instillation.2,14 But, these agents do not display any greater efficacy in extinguishing the T-cell-mediated responses associated with GPC than other mast cell-stabilizing drugs.2
Topical non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids represent two additional drug classes that have been used with some success. NSAIDs have been referred to as the first drug of choice for conjunctival inflammation.2 Their mechanism of action involves blockading the cyclo-oxygenase arm of the arachidonic acid pathway, which inhibits the formation of pro-inflammatory mediators such as prostaglandins and thromboxanes. In addition, NSAIDs (e.g., ketorolac, nepafenac and bromfenac) are believed to increase the threshold of sensory nerves, helping to alleviate discomfort associated with various allergic and inflammatory conjunctivitides.2
But, despite these attributes, few practitioners seem to rely on NSAIDs as a primary therapy for CLPC. This may stem from the fact that NSAIDs, like mast-cell stabilizers, can have a delayed onset of action and limited efficacy against CLPC.15
Corticosteroids are probably the most comprehensive therapeutic option in CLPC. Steroids completely inhibit the arachidonic acid pathway, preventing both prostaglandin and leukotriene synthesis by blocking the action of phospholipase A2. In addition, these drugs have the capacity to stabilize cell membranes, so they may diminish mast cell degranulation.16
But, the use of topical steroids as first-line agents in ocular allergy managementas well as CLPCremains controversial due to the potential side effects of long-term steroid use. These include elevated intraocular pressure, cataractogenesis, and increased susceptibility to ocular infection. For this reason, corticosteroids should only be used for short-term management of CLPC. Ester-based steroids, such as loteprednol, have also been widely advocated over ketone-based steroids (e.g., prednisolone acetate or fluorometholone) in cases of CLPC because they have a greater safety profile with regard to the aforementioned side effects.16-18
Other treatment modalities for CLPC have been employed with limited or anecdotal success. Topical cyclosporine A offers a long-term immunomodulatory option without the potential problems associated with corticosteroids. Unfortunately, cyclosporine tends to sting somewhat upon instillation, and does not provide rapid relief. Most often, this medication is employed following a short course of steroids, and is continued as maintenance therapy for patients with recalcitrant disease. Although there are currently no peer-reviewed data in the literature that supports cyclosporine for GPC management, its utility in the treatment of other forms of allergic conjunctivitis is well-established.19 Similarly, another immunomodulatory agenttopical tacrolimus ointmenthas recently been shown to be effective in refractory CLPC.20
Primary management of CLPC should include reduced exposure to the offending substrate. Increased frequency of contact lens cleaning, enzymatic removal of proteins, and/or frequent replacement is paramount, as is providing the patient with the best and healthiest fitting options. However, topical medications provide an effective adjunct to this therapy, and can hasten the resolution of this elusive disorder. Understanding these treatment options is our best defense against this giant problem.
1. Donshik PC, Ehlers WH, Ballow M. Giant papillary conjunctivitis. Immunol Allergy Clin North Am 2008 Feb;28(1):83-103.
2. Stapleton F, Stretton S, Sankaridurg PR, et al. Hypersensitivity responses and contact lens wear. Cont Lens Anterior Eye 2003 Jun;26(2):57-69.
3. Begley CG, Riggle A, Tuel JA. Association of giant papillary conjunctivitis with seasonal allergies. Optom Vis Sci 1990 Mar;67(3):192-5.
4. Allansmith MR, Baird RS. Percentage of degranulated mast cells in vernal conjunctivitis and giant papillary conjunctivitis associated with contact-lens wear. Am J Ophthalmol 1981 Jan;91(1):715.
5. Korb DR, Greiner JV, Finnemore VM, Allansmith MR. Biomicroscopy of papillae associated with wearing of soft contact lenses. Br J Ophthalmol 1983 Nov;67(11):733-6.
6. Suchecki JK, Donshik P, Ehlers WH. Contact lens complications. Ophthalmol Clin North Am 2003 Sep;16(3):471-84.
7. Donshik PC. Giant papillary conjunctivitis. Trans Am Ophthalmol Soc 1994; 92:687-744.
8. Skotnitsky CC, Naduvilath TJ, Sweeney DF, et al. Two presentations of contact lens-induced papillary conjunctivitis in hydrogel lens wear: local and general. Optom Vis Sci 2006 Jan;83(1):27-36.
9. Skotnitsky C, Sankaridurg PR, Sweeney DF, Holden BA. General and local contact lens induced papillary conjunctivitis (CLPC). Clin Exp Optom 2002 May;85(3):193-7.
10. Meisler DM, Berzins UJ, Krachmer JH, Stock EL. Cromolyn treatment of giant papillary conjunctivitis. Arch Ophthalmol 1982 Oct;100(10):1608-10.
11. Allansmith MR, Ross RN. Ocular allergy and mast cell stabilizers. Surv Ophthalmol 1986 Jan-Feb;30(4):229-44.
12. Lustine T, Bouchard CS, Cavanagh HD. Continued contact lens wear in patients with giant papillary conjunctivitis. CLAO J 1991 Apr;17(2):104-7.
13. Schmid KL, Schmid LM. Ocular allergy: causes and therapeutic options. Clin Exp Optom 2000 Sep;83(5):257-70.
14. Bielory L. Ocular allergy guidelines: a practical treatment algorithm. Drugs 2002;62(11):1611-34.
15. Friedlaender MH. Contact lens induced conjunctivitis: a model of human ocular inflammation. CLAO J 1996 Jul;22(3):205-8.
16. Ilyas H, Slonim CB, Braswell GR, et al. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens 2004 Jan;30(1):10-3.
17. Friedlaender MH, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. The Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group
18. Asbell P, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. CLAO J 1997 Jan;23(1):31-6.
19. Daniell M, Constantinou M, Vu HT, Taylor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol 2006 Apr;90(4):461-4.
20. Kymionis GD, Goldman D, Ide T, Yoo SH. Tacrolimus ointment 0.03% in the eye for treatment of giant papillary conjunctivitis. Cornea 2008 Feb;27(2):228-9.

