 |
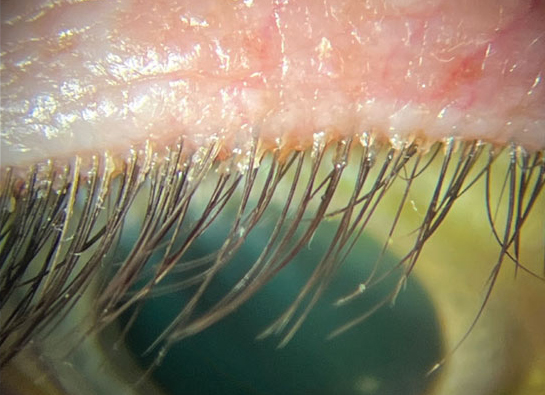
Reduction in collarettes at the base of lashes, number of Demodex tails observed and lash follicle pouting demonstrated the effects of ivermectin cream nightly treatment. Photo: Cecelia Koetting, OD. Click image to enlarge. |
With the recent approval of Xdemvy (lotilaner ophthalmic solution 0.25%, Tarsus Pharmaceuticals), treatment of Demodex blepharitis has gained attention as an intervention worth considering in viable candidates. However, the therapy requires a six-week course of treatment and involves one drop of the solution in each eye, administered twice daily approximately 12 hours apart. While clinical results with this drug are promising and suggest that most patients will benefit from the eye drop, optometrists must be prepared to care for patients who don’t see full benefit after the six-week treatment period.
Topical ivermectin, an antiparasitic drug which has been approved for the treatment of Demodex infestation associated with rosacea, has also shown recent clinical success in retrospective studies involving ocular Demodex blepharitis, although it is an off-label therapy and not currently approved for ocular application. A recent retrospective study investigated the treatment efficacy of nightly application of topical ivermectin 1.0 % cream for three months on alleviating clinical signs of ocular demodicosis and whether any potential improvements are sustained over a longer period of up to 12 months. The research team determined that the treatment reduced the irritating condition signs and sustained that effect up to one year. They believed it was made evident through the improvement in the signs of ocular demodicosis, including reduction in collarettes at the base of lashes, number of Demodex tails observed and lash follicle pouting.
The retrospective single-center clinical practice chart analysis involved the off-label treatment of patients who had ocular demodicosis with topical ivermectin 1.0% cream (Soolantra, Galderma) applied nightly to the lid margins of both eyes for three months. Ocular surface health was assessed at baseline when the treatment was prescribed and followed up at three and 12 months after baseline. Slit lamp biomicroscopy was used to take digital images of the upper eyelid lashes. Manual image analysis was conducted to quantify signs of ocular demodicosis including the number of lashes with collarettes, with visible Demodex tails and with follicle pouting. Data from a total of 75 patients with ocular demodicosis was analyzed for this study (mean age 66.6 years, 44 female).
The median numbers of lashes with collarettes (eight at baseline to zero at the final visit) and lashes with follicle pouting (three at baseline to zero at the final visit) decreased with treatment. Any sign of lashes with visible tails was eliminated by the final visit. By alleviating the Demodex load, ocular surface health improved with topical ivermectin treatment. Fluorescein staining severity score also improved, particularly from baseline (one) to the second visit (zero). Notably, no serious side effects were observed in any of the patients with only mild irritation reported.
“The treatment protocol in the current study enables therapy to be conducted at home, reducing chair time compared to in-office application of cream,” the authors noted in their paper. “However, clinicians should be cautious of the use of ivermectin in patients on anticoagulant therapy as there is a potential for ivermectin to interfere with these therapies.”
The researchers found that a potential benefit of assessing lash pouting is that it is an assessable sign in patients who may still have high Demodex load but have low number of collarettes due to good lid hygiene.
“Demodex tail quantification may have been even more apparent with an additional diagnostic technique using eyelash manipulation by applying lateral tension with forceps without epilation, which could be a consideration for future studies,” the team added.
They concluded that their study shows evidence of good efficacy and safety profile of topical ivermectin 1.0 % cream in alleviating signs of ocular demodicosis and improving ocular surface health in affected patients.
Smith M, Wolffsohn JS, Chiang JCB. Topical ivermectin 1.0% cream in the treatment of ocular demodicosis. Cont Lens Anterior Eye. December 3, 2023. [Epub ahead of print]. |

