Meibomian gland dysfunction (MGD) is now considered the leading cause of dry eye.1 This is according to the published findings of the prestigious 2011 International Workshop on MGD.1 Conceptually, this is a substantial shift in our thinking, leading us away from the traditional inflammation-driven models of dry eye disease etiology. A review of the literature reveals that our unwavering focus on inflammation as the driving force behind dry eye, in retrospect, has been a distraction for some time.
While identifying and managing ocular surface inflammation is critical, we will never fully understand dry eye if we remain fixated on treating inflammation alone. If we turn our attention to what is causing the inflammation, we can open our minds to the recent paradigm shift—that MGD is currently thought to be the leading cause of dry eye.1 With this new foundational thinking, our approach to diagnosis and management must necessarily undergo a makeover. To begin, we will review MGD and how to evaluate meibomian gland health.
Changes in Diagnosis
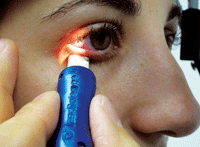
The Meibomian Gland Evaluator (MGE, TearScience Inc.) in use during the diagnostic evaluation of habitual meibomian gland function.
The historical and narrow concept of MGD as a hypersecretory disorder with obvious signs of infection and inflammation has been expanded to include the much more commonly encountered obstructive hyposecretory and often non-obvious form of MGD. This form of MGD results in inadequate lipid production for the formation of the oily layer of the tears.1 Obstructive MGD is the most common form of MGD resulting in dry eye.2
In the recent past, a clinical diagnosis of MGD has been based primarily on overt signs of morphological changes of the lid margins and meibomian gland orifices. For example, these include pouting (an elevated internal plug of solidified secretions, which may be expressed from the orifice with pressure); capping (a dome over the orifices possibly of solidified oil that may or may not be epithelialized); reduction in number of the orifices and loss of definition of the orifice cuffs; erythematous, irregular, thickened lid margins, with or without telangiectasia surrounding orifices; and serration of the lid margin.3
Conversely, non-obvious and early obstructive MGD presents a more tricky diagnosis, given that very few or even none of the aforementioned obvious clinical signs are observable with a slit lamp. We now believe that there is only one way to diagnose MGD under these circumstances, and that is to test the functionality of the meibomian glands directly.4
If the glands are not functional and the patient is symptomatic, regardless of the appearance of the lids and ocular surface, this should direct our treatment towards rehabilitating the glands. This holds true even in the relatively rare case of dry eye that may have originated as true aqueous deficient dry eye (ADDE).
Meibomian Gland Functionality
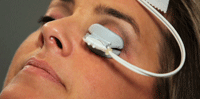
The LipiFlow (TearScience Inc.) during a 12-minute, in-office dry eye treatment. It simultaneously applies heat and pulsatile pressure to the eyelids.
What is the best way to assess meibomian gland function? The brute-force digital methods of the past have been effective at diagnosing the old medical model form of infective MGD, where the glands are full of turbid thick secretions that can only be expressed with force. There are many ways to grade these secretions and, while important, they do little to inform us of the habitual function of the glands on a daily basis. What we really want to know is whether the glands release functional oil during everyday blinking.
A recent innovation by TearScience Inc. known as the Meibomian Gland Evaluator now offers a metric to standardize the amount of force applied to the glands during diagnostic testing and to mimic the force experienced by the glands during a deliberate blink.5 Because this force is much less than the force of the typical digital manipulation of the glands used in the past, the amounts of oil that are expressed are very small and require careful inspection.
Once we have gained some knowledge of the patient’s meibomian gland function through evaluation and other more familiar testing (e.g., tear film break-up time, symptom report, ocular surface staining/staining the lid wiper, Schirmer testing, etc.), we can proceed with a treatment plan. From the work of Anthony Bron, M.D., F.R.C.Ophth., we know that while dry eye can be academically classified as ADDE or evaporative dry eye (EDE) in nature, both forms become virtually indistinguishable as the disease progresses, rendering attempts to make the distinction fairly meaningless.6
Even if we are able to clearly and predictably distinguish ADDE from EDE, dry eye presents such a clinical conundrum with tremendously high treatment failure that the accepted clinical approach to therapy remains one of “use whatever works.” Options are: tear replacement or supplementation, ocular lubricants, anti-inflammatory therapy, immune modulators, lid therapy, environmental adjustments (including humidity goggles) and meibomian gland rehabilitation. All of these are intended to improve, maintain or protect the health of the ocular surface, regardless of the disease etiology.
So, which treatment path is likely to give us the highest probability of short- and long-term success? Because MGD currently is understood to be the leading cause of dry eye, it makes sense to start there; but all categories of treatments will be mentioned as they all, potentially, serve a role in our clinics.
Treatment for MGD and Dry Eye
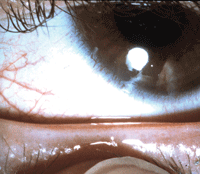
A normal healthy eyelid. Upon gentle pressure over the glands, the gland orifices release clear liquid oil.
Treatment of MGD is designed to restore the normal flow of meibomian gland secretions, thereby increasing the likelihood of a healthy lipid layer and consequently reducing tear evaporation and enhancing tear film stability. This outcome is achieved primarily through removal of material obstructing the ducts of the glands. The four main treatment approaches for MGD are as follows: 1) physical expression to remove obstruction material; 2) application of heat to the eyelids to soften and preferably liquefy solidified and obstructive meibomian gland contents; 3) lid scrubs to relieve external meibomian gland orifice blockage due to epithelial overgrowth; and 4) medications to mitigate infection and inflammation of the eyelids and also to possibly improve the lipid profile of the glands.
Let’s look at each treatment approach in turn:
• Physical expression. Physical expression of meibomian glands for therapeutic purposes is an in-office procedure that has at least an 80-year history and can be supplemented by the patient performing self-expression at home.7 The reported techniques vary from gently massaging the lids against the eyeball to forceful squeezing of the lids either against each other or between a rigid object on the inner lid surface and a finger, thumb or rigid object (glass rod, cotton swab, metal paddle, etc.) on the outer lid surface.8 The purpose of the rigid object on the inner lid surface is to protect the eyeball from forces transferred through the eyelid, and also to offer stable resistance to increase the amount of force that can be applied to the glands.
The amount of force needed to express obstructed glands can be significant. Unfortunately, the pain frequently is also significant and typically this is the limiting factor in the overall efficacy of the technique.8 A recent study reported that just 7% of the patients could tolerate the pain resulting from adequate therapeutic physical expression of the glands.8
Regardless of the method of meibomian gland physical expression, the goal is to evacuate the obstruction and other meibomian gland material from the gland, thereby creating a new environment that offers the potential for normal gland secretion. If physical expression is a viable option, it should be continued until the dysfunction is resolved.7
• Heat therapy. The application of heat to the eyelids has traditionally been implemented by using warm compresses. However, many innovative devices have emerged with the goal of providing a regulated elevated temperature to the eyelids. These tools, in the forms of a face-mask, goggle or similar device, have been designed to more effectively heat the lids and increase convenience for the patient, possibly improving compliance.9,10
These devices are not subject to FDA approval and typically are home therapies that patients would purchase for themselves. While difficult to manage from a compliance and safety perspective, the at-home therapies can be effective and should be recommended for patients that are willing and able to use them safely.9 It is important to note that the efficiency of all techniques where heat is applied to the outer lid surfaces are limited by the diffusion of heat through the lid tissue and tarsal plate, and also the vascular effect of the blood flow transporting the heat away from the lids.
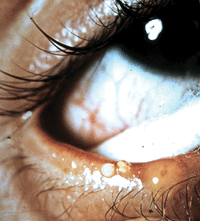
A lid with MGD (obvious signs are telangiectasia, lid margin thickening and posterior blepharitis). Diagnosis can be made by observation of the lid margin. Dysfunctional glands release a thick opaque material upon forceful expression.
When we prescribe home therapy in the form of warm compresses or similar heating methods, it is critical to inform the patient not to directly place pressure over the cornea and also to avoid any eye rubbing following heat therapy. Recent studies have indicated that home heat therapy can raise the corneal temperature. This alone, or in combination with physical manipulation of the cornea through massage or eye rubbing, can lead to transient visual distortions or even keratoconus in apparently normal eyes.11,12
The use of intense pulsed light (IPL) therapy has been suggested as an off-label treatment for MGD. However, there are currently no peer-reviewed studies published on this technique, and therefore little is known about the precise mechanism of action for the treatment of MGD or the safety risks of repeated treatment. We will know more when safety and efficacy have been investigated.
• Simultaneous heat and expression. As of August 2011, there is one FDA-approved treatment for EDE involving the simultaneous application of heat and pulsatile pressure to the eyelids. The treatment device is called the LipiFlow, manufactured by TearScience Inc.13 It is being used in academic centers and private practices in the United States, Canada and Europe, and has been shown to improve both meibomian gland functionality and reduce dry eye symptoms in multicenter studies.13-15
The LipiFlow treatment is unique and significantly more effective than any other available heating method.13 The heat is applied to the inner lid surface directly over the glands, thus avoiding the heat loss incurred with conventional methods as the heat diffuses through the outer lid tissue and the tarsal plate prior to reaching the glands. The lids are sandwiched between the heater on the inner lid surfaces and a pulsatile pressure component on the outer lid surfaces, which allows for expression of the gland contents of upper and lower eyelids simultaneously during the 12-minute heating phase. This is in contrast to the conventional therapeutic gland expression that can only occur after the heating phase. In addition, because the gland expression occurs during heating, there is no pain experienced during the administration of the treatment.13,14
Although there is no single melting point for solidified meibomian secretion because the chemistry and viscosity of the solidified secretion itself are variable, we know that solidified secretions from severely obstructed glands have a considerably higher melting point than those from apparently normal, unobstructed glands.16,17 So, it can be assumed that higher temperatures, provided safety is maintained, are superior for the treatment of more severely obstructed glands.
• Lid scrubs. Lid scrubs, although primarily suggested for anterior blepharitis, can help remove crusts and inspissated secretions blocking the gland orifices. Lid scrubs are performed on the lid margin, over the meibomian gland orifices to prevent epithelial overgrowth or obstructive material from sealing the orifice. Use of lid scrubs and lid massage also have been shown to improve tear film break-up time, and lid hygiene also helps and is the universal treatment for any associated staphylococcal or seborrheic blepharitis. Therefore, use of lid scrubs has been suggested as part of the daily routine of heat therapy and lid massage in patients with MGD.9
• Intraductal probing. An alternative to the more conventional heat and expression is physical probing of each individual meibomian gland orifice. A single study reported short-term relief of symptoms and reduction in the inflammatory signs of obstructive MGD as a result of intraductal gland probing.18 Future investigations will expand our knowledge of the safety and efficacy of this treatment in the short and long term.
• Medication. Antibiotics—particularly the tetracyclines, including doxycycline, tetracycline and minocycline—continue to find a place in the modern management of MGD. Tetracyclines decrease the secretion of bacterial lipases that are known to break down the normal meibum lipids into inflammatory free fatty acid fragments.19 In addition, tetracyclines are said to have anti-collagenase and anti-matrix metalloproteinase (MMP) properties.20,21
Success also has been reported with minocycline in decreasing a branch chain fatty acid to its normal values, resulting in an increase in tear film break-up time following two months of minocycline therapy in patients with MGD.22 Other studies have shown the benefits of doxycycline over tetracycline.23 Although the involved mechanisms may not be fully understood, systemic tetracyclines appear to improve the lipid profile of meibomian gland secretion.9
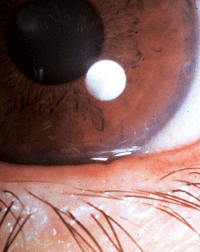
A “quiet” lid margin with MGD. The only visible sign is lid margin serration. The MGD is not obvious. This patient is highly symptomatic with significant obstructive MGD. Diagnosis is made upon meibomian gland expression.
Similar to the anti-inflammatory properties of tetracyclines, macrolides also have anti-inflammatory and anti-MMP activity.24 The topical drug treatment efficacy of azithromycin (1% and 1.5%) has shown some success in patients with MGD.25,26
While there is evidence that antibiotics may improve patient symptoms and meibomian gland lipid secretion quality, there is no evidence that antibiotics can relieve meibomian gland obstruction. This suggests that the improvements may not be in functional restoration of obstructed meibomian glands, but in better quality of meibomian gland secretions. If the latter is correct, antibiotic therapy may be very beneficial if administered in combination with, or immediately after, treatment to remove the meibomian gland obstruction (physical expression, heat therapy, etc.).
The use of topical cyclosporine, an immunosuppressant, in ADDE (especially with ocular surface inflammation) has been established.27 There is also some supporting evidence for its role in MGD in the form of improvement in objective signs and sometimes, also symptoms.27,28 Similar to the use of antibiotics, once gland obstructions have been removed via heat therapy and physical expression, topical cyclosporine may then help alleviate any associated inflammatory components.
The administration of topical corticosteroids to suppress the inflammatory response associated with dry eye has been shown to be effective in the relief of dry eye signs; reduction in ocular surface staining; reduction of lid wiper epitheliopathy and dry eye symptoms.29-32 The use of steroids usually is temporary or pulsatile due to the potential risks of chronic use. Additionally, the obstruction would first have to be removed from the glands, in conjunction with steroid use, to increase the likelihood of treatment success.
Topical androgens continue to be investigated for the treatment of dry eye and MGD but further research is required to elucidate their role in the clinic.33,34
• Diet. Dietary alteration and supplementation has been gathering support in recent years. Because patients with MGD have an altered lipid composition, changing the dietary lipid intake may affect the lipid composition of the meibomian glands. As such, omega-3 supplements have been recommended as treatment for dry eye and MGD.35 There is also recent evidence that omega-3 consumption may significantly reduce certain conjunctival inflammatory markers.36 Thus, published data provide some evidence to recommend dietary alterations and supplementation of omega-3; however, further research is required to fully quantify or evaluate these effects.9
• Tear supplementation. Tear supplementation remains a mainstay in the management of dry eye. Tear substitutes perform many important roles, such as improving the optical quality of the tear film, alleviating dry eye symptoms, facilitating adequate spreading of meibomian gland secretions, and increasing lubrication between the eyelid wiper and the ocular surface, to name just few. Not surprisingly, the newer tear substitutes that incorporate lipid emulsions demonstrate significant improvements in tear stability, patient comfort and lipid layer thickness.37-39
One can also apply ointment to the lid margins, providing a reservoir of lipid on the eyelid to replenish the tear film lipids upon blinking. The only issue with this is that the ointment typically results in an annoying transient blurring of vision and is therefore best applied at night.40
• Environmental modifications. Environmental modifications will have a significant effect on patient symptoms because exposure to conditions of low relative humidity and temperature-controlled heated or air-conditioned environments increase evaporative tear loss.
This aspect of patient education regarding dry eye management is probably underemphasized. The tempering of our living and working conditions, where possible, to increase the relative humidity can dramatically reduce symptoms and the need for tear supplements. The successful use of moisture goggles demonstrate this effect.9,41
In conclusion, the past several years have seen a remarkable paradigm shift in our emphasis from an inflammatory-driven, aqueous deficient model of dry eye to one of lipid-deficient evaporative dry eye as a sequela of MGD. Thus, our focus now targets the eyelids and meibomian glands rather than the lacrimal gland output. Our experience confirms the conclusion of the prestigious MGD workshop that MGD is likely the leading cause of dry eye throughout the world. Emerging therapies for dry eye will further reflect this thinking. In the meantime, we can sharpen our observational skills as they pertain to the eyelids, the lid margins and the meibomian glands themselves.
Dr. Blackie is an optometrist in Boston and is the clinical research scientist for TearScience Inc., in Morrisville, N.C. Dr. Korb is in private practice at Korb Associates, Boston, and is the co-founder and chief technical officer of TearScience Inc.
Both authors have disclosed that they receive financial support from Korb Associates and TearScience Inc. However, neither author solicited exposure. Rather, they were invited by Review to discuss this technology based on their experience.
1. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1922-9.
2. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1930-7.
3. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003 Jul;1(3):107-26.
4. Blackie CA, Korb DR, Knop E, et al. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010 Dec;29(12):1333-45.
5. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008 Dec;27(10):1142-7.
6. Bron AJ, Yokoi N, Gafney E, et al. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf. 2009 Apr;7(2):78-92.
7. Duke-Elder WS, MacFaul PA. The Ocular Adnexa, Part II, Diseases of the Eyelids. Vol. 13. In: Kimpton H (ed). System of Ophthalmology. London:1974:242.
8. Korb DR, Blackie CA. Meibomian gland therapeutic expression: quantifying the applied pressure and the limitation of resulting pain. Eye Contact Lens. 2011 Sep;37(5):298-301.
9. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):2050-64.
10. Blephasteam: Information. Available at: www.blephasteam.com/info.htm (accessed April 18, 2012).
11. Solomon JD, Case CL, Greiner JV, et al. Warm compress induced visual degradation and Fischer-Schweitzer polygonal reflex. Optom Vis Sci. 2007 Jul;84(7):580-7.
12. McMonnies CW, Korb DR, Blackie CA. The role of heat in rubbing and massage-related corneal deformation. Cont Lens Anterior Eye. 2012 Feb 4. [Epub ahead of print]
13. Lane SS, Dubiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012 Apr;31(4):396-404.
14. Friedland BR, Fleming CP, Blackie CA, Korb DR. A novel thermodynamic treatment for meibomian gland dysfunction (MGD). Curr Eye Res. 2011 Feb;36(2):79-87.
15. Greiner JV. A single LipiFlow thermal pulsation system treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Current Eye Research. 2012 Feb 10. [Epub ahead of print]
16. Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004 Mar;78(3):347-60.
17. Blackie CA, Solomon JD, Greiner JV, et al. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008 Aug;85(8):675-83.
18. Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010 Oct;29(10):1145-52.
19. Dougherty JM, McCulley JP, Silvany RE, et al. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991 Oct;32(11):2970-5.
20. Golub LM, Ramamurthy NS, McNamara TF, et al. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2(3):297-321.
21. Li DQ, Lokeshwar BL, Solomon A, et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001 Oct;73(4):449-59.
22. Souchier M, Joffre C, Gregoire S, et al. Changes in meibomian fatty acids and clinical signs in patients with meibomian gland dysfunction after minocycline treatment. Br J Ophthalmol. 2008 Jun;92(6):819-22.
23. Yoo SE, Lee DC, Chang MH. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol. 2005 Dec;19(4):258-63.
24. Ianaro A, Ialenti A, Maffia P, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000 Jan;292(1):156-63.
25. Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther. 2008 Sep;25(9):858-70.
26. Foulks GN, Borchman D, Yappert M, et al. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010 Jul;29(7):781-8.
27. Perry HD, Doshi-Carnevale S, Donnenfeld ED, et al. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006 Feb;25(2):171-5.
28. Rubin M, Rao SN. Efficacy of topical cyclosporin 0.05% in the treatment of posterior
blepharitis. J Ocul Pharmacol Ther. 2006 Feb;22(1):47-53.
29. Avunduk AM, Avunduk MC, Varnell ED, et al. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003 Oct;136:593-602.
30. Sainz de la Maza Serra M, Simón Castellvi C, Kabbani O. Nonpreserved topical steroids and punctal occlusion for severe keratoconjunctivitis sicca. Arch Soc Esp Oftalmol. 2000 Nov;75(11):751-6.
31. Hyon JY, Lee YJ, Yun PY. Management of ocular surface inflammation in Sjögren syndrome. Cornea. 2007 Oct;26:S13–15.
32. Herman JP, Korb DR, Greiner JV et al. Treatment of lid wiper epitheliopathy with a metastable lipid emulsion or a corticosteroid. IOVS. 2005;46:S2017.
33. Sullivan BD, Evans JE, Krenzer KL, et al. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000 Dec;85(12):4866-73.
34. Krenzer KL, Dana MR, Ullman MD, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab 2000 Dec;85(12):4874-82.
35. Paranjpe DR, Foulks GN. Therapy for meibomian gland disease. Ophthalmol Clin North Am. 2003 Mar;16(1):37-42.
36. Brignole-Baudouin F, Baudouin C, Aragona P, et al. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011 Nov;89(7):e591-7.
37. Scaffidi RC, Korb DR. Comparison of the efficacy of two lipid emulsion eyedrops in increasing tear film lipid layer thickness. Eye Contact Lens. 2007 Jan;33(1):38-44.
38. Di Pascuale MA, Goto E, Tseng SC. Sequential changes of lipid tear film after the instillation of a single drop of a new emulsion eye drop in dry eye patients. Ophthalmology. 2004 Apr;111(4):783-91.
39. Solomon R, Perry HD, Donnenfeld ED, et al. Slit lamp biomicroscopy of the tear film of patients using topical Restasis and Refresh Endura. J Cataract Refract Surg. 2005 Apr;31(4):661-3.
40. Goto E, Shimazaki J, Monden Y, et al. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology. 2002 Nov;109(11):2030-5.
41. Korb DR, Greiner JV, Glonek T, et al. Effect of periocular humidity on the tear film lipid layer. Cornea. 1996 Mar;15(2):129-34.

