A 58-year-old black male presented with a family history of glaucoma (a sibling). Upon examination, his intraocular pressure measured 21mm Hg O.D. and 22mm Hg O.S. His pachymetry readings were 483µm O.D. and 481µm O.S. Gonioscopy revealed open angles O.U.
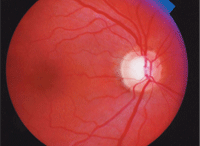
The big question: Will this patient convert to
glaucoma? And if so, then what?
Dilated fundus examination revealed moderate-sized cups with mild vertical elongation of both cups and a disc hemorrhage in the left eye. The results of automated static threshold perimetry revealed no glaucomatous defects in either eye, but the confocal scanning laser ophthalmoscope findings were borderline, with cup-shape measures of -0.18 O.D. and -0.14 O.S., and mean retinal nerve fiber layer of 0.20mm O.D. and 0.18mm O.S.
So, where does this patient stand?
Clinicians are faced with this question daily: Is my patient a glaucoma suspect? If you deem your patient to be a glaucoma suspect, the next question is: Will the patient convert to glaucoma and when do I initiate treatment?
The clinical care of glaucoma suspects and patients involves an analysis of risk assessment and risk management. The ultimate goal is to preserve vision in a manner that allows the patient to be an active participant in their own care along with treatment that minimizes the negative effects on quality of life, including financial. For instance, a study in 2006 reported the direct cost of a patient diagnosed as a glaucoma suspect was $623/year compared to a patient with end-stage disease with direct cost of $2,511/year.1
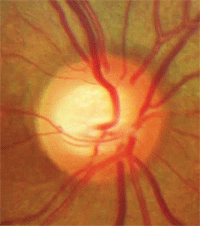
Vessel baring.
When to Be Suspicious
A glaucoma suspect has significant risk factors for the development of glaucoma or signs consistent with glaucoma. The major risk factors (in regard to history and demographics) are age, race and family history. High myopia, diabetes, hypertension, trauma, pseudoexfoliation, pigmentary dispersion syndrome and sleep-related breathing disorders are other potential threatening associations.
The most common initial signs of glaucoma during routine examination are elevated intraocular pressure (IOP) and changes or abnormalities in optic nerve head (ONH) appearance.
(We’ll address elevated IOP below.) In regard to evaluating the ONH, be attentive to these important signs in patients at risk for glaucoma:
• Thinning of the optic nerve rim. A “normal” optic nerve follows the “ISNT” rule. The “ISNT” rule asserts that the typical optic nerve rim presents
with the inferior rim the thickest, followed by the superior rim, the nasal rim and finally the temporal rim as the thinnest. Deviation from the ISNT rule is a risk factor for development of glaucoma. Glaucomatous cupping usually affects the superior and inferior rims to a greater extent.2 This results in inferior and superior rim thinning, which alters the “expected” ISNT ONH appearance.
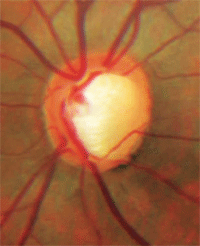
Notching of the neuroretinal rim with a nerve fiber layer defect.
• Cupping extending beyond the vascular trunk. The major vessels entering the eye are typically abutting the rim tissue, meaning that those vessels run just along the inside of the cup and are supported by rim tissue. If the vascular trunk is not located directly next to the rim tissue, there is a possibility that extension of the optic nerve cup has occurred and the loss of nerve fibers now leaves the vascular trunk standing free.
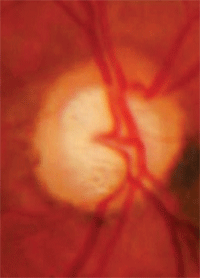
Cupping that extends beyond the
vascular trunk, with an ONH nevus.
• Asymmetry of C/D. Glaucoma is often a bilateral, asymmetric condition. So be suspicious if a larger cup-to-disc ratio is present in one eye with symmetrical disc size. Consideration must also be given to unilateral megalopapilla, which is defined as an optic nerve surface area greater than 2.5mm2.3 These patients have a large cup in a large disc but have normal rim tissue.
• Vessel baring. Vessels running horizontally that were initially supported by rim tissue may have underlying tissue loss, leaving a vessel suspended over free space. This condition of a suspended vessel is described as vessel baring.
• “Bean-potting” (shelving). Bean-potting, also described as shelving, tends to occur later in glaucoma and is described as vessels visibly entering the optic nerve cup over the rim and then disappearing behind the excavated tissue and re-emerging at the bottom of the cup.
• Notching of the rim. Notching of the neuroretinal rim is a sign that a focal area of loss occurred within the rim tissue. Notching most commonly occurs at the inferior temporal or superior temporal aspect of the disc and often is associated with corresponding nerve fiber layer defects.4 Optic nerve rim notching is believed to be secondary to ischemia.
• Disc hemorrhage. A disc hemorrhage is also believed to occur as a result of ischemia. A disc hemorrhage is defined as a hemorrhage within one disc diameter of the optic nerve.
Not every patient with glaucoma presents with each of these signs, but close examination of the optic nerve generally reveals some of these defects in patients with pathology.
Moreover, some of these signs, such as a disc hemorrhage, indicate not only that the patient likely has glaucoma or sub-clinical glaucoma, but also that the disease is poorly controlled and additional therapy or improved compliance is warranted.5
To Treat or Not to Treat?
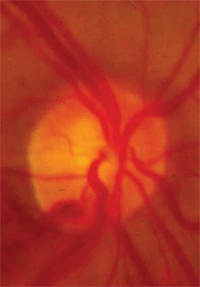
Disc hemorrhage.
The decision to treat or not treat a patient with suspicious glaucoma findings rests on an assessment of the risk factors, combined with the results of a complete glaucoma evaluation.
Most glaucoma evaluations occur over several visits, after which the clinician makes an initial status assessment for the glaucoma diagnosis. These initial evaluations are also important for establishing baseline data for a number of tests for comparison at subsequent visits to determine if the person is either a glaucoma suspect or a patient with glaucoma.
The most common test involved in the glaucoma evaluation is intraocular pressure, measured on several visits and at several times of the day (serial tonometry). Measurement of central corneal thickness is also completed; most commonly it’s measured by pachymetry, although newer technology such as anterior segment OCT may also be applied.
Stereoscopic optic nerve photo documentation is performed initially to establish a baseline and at regular intervals (typically yearly) to monitor for progression.
Gonioscopy is done to ensure open angles and to determine the presence of secondary glaucoma findings. Automated static threshold perimetry is completed to establish baseline visual field status and look for characteristic defects consistent with glaucoma. If available, diagnostic laser imaging may also be used to gather baseline data and look for characteristic glaucomatous patterns.
Ocular blood flow may also be performed, but to date it is considered an additional tool.
Let’s consider each of these tests in turn:
• Tonometry. While important in determining the presence and in the management of glaucoma, the intraocular pressure alone does not make or exclude a diagnosis of glaucoma or dictate treatment. Prior to understanding the importance of central corneal thickness (CCT), clinicians typically had a specific IOP—a “magic number”—above which they would initiate treatment, such as 30mm Hg, regardless of the other ocular findings.

Angle recession shown by gonioscopy.
Today, that approach is tempered with the results of pachymetry and, for many clinicians, there is no single number at which they initiate treatment. The Ocular Hypertensive Treatment Study (OHTS) used 24mm Hg as the point to initiate treatment in the study design. But, as the study demonstrated, CCT played a significant role in the progression from suspect to glaucomatous change.6 In addition to individual IOP measurements, it is important to find the individual’s range of values and identify patients who have a larger diurnal curve. A large curve, as well as asymmetric IOPs, can contribute to a greater risk.
• Pachymetry. The importance of CCT measured with pachymetry has been reviewed extensively since OHTS. Indeed, OHTS showed that CCT has been identified as an independent risk factor beyond just an IOP conversion factor. Most clinicians use pachymetry in general terms by identifying the value simply as “thick,” “thin” or “normal,” with the greatest risk of development of glaucoma for patients with thin corneas. Be mindful that inexperienced operators may produce a thinner value than actual because they exert too much force when performing pachymetry.
• Gonioscopy. This is an underutilized tool in the management and identification of glaucoma. When performed, gonioscopy can ensure that the ocular drainage angle is open and identify underlying secondary glaucoma components that may assist in confirming a diagnosis as glaucoma suspect or glaucoma patient. Common secondary glaucoma findings include excessive pigmentation (e.g., pigment dispersion or pseudoexfolation), angle recession and peripheral anterior synechiae (e.g., traumatic glaucoma or past inflammation) or neovascularization from underlying ischemic conditions.

Visual field defects that suggest glaucoma include nasal step, arcuate scotoma, enlarged blind spot and paracentral scotoma (such as this one shown here).
Less common conditions associated with glaucoma development may also be identified by gonioscopy, such as the iridocorneal endothelial (ICE) syndromes and anterior cleavage syndrome.
• Perimetry. Automated static threshold perimetry establishes baseline visual status and can identify characteristic visual field defects that help determine patient status. Visual field defects most consistent with glaucoma include nasal step, arcuate scotoma, enlarged blind spot and paracentral scotomas. Realize that not many patients can do a reliable visual field in one attempt and that a visual field is not a useful tool unless it is reliable.
• Nerve fiber layer analysis. Nerve fiber analysis can be done in the office with a red-free filter and high illumination evaluation of the optic nerve with the fundus lens (e.g., 90D, 78D, 66D) evaluation and/or with careful analysis of optic nerve photos. More detailed information is available with diagnostic laser imaging of the optic nerve, rim and nerve fiber layer. While diagnostic laser imaging is not required at this point to maintain the standard of care, these devices are beneficial in early diagnosis of nerve fiber layer loss and/or early optic nerve progression.
Nerve fiber analysis can be mechanically measured in a variety of ways, such as with automated measures on scanning laser polarimeter or in monitoring for progression with the confocal scanning laser ophthalmoscope. In addition to ONH and nerve fiber analysis, macular retinal thickness may be beneficial in the future for early detection of glaucoma.7
Monitoring
Upon completion of a glaucoma evaluation, the clinician needs to decide whether the patient has glaucoma, and whether to initiate treatment based on the findings.
If a glaucoma evaluation does not compel the clinician to initiate treatment, then the patient is deemed a glaucoma suspect and is monitored for progression. A glaucoma suspect can further be categorized into a low, medium and high risk.
Remember that a patient considered a glaucoma suspect is labeled as such because of the likelihood for progression; therefore the clinician needs to be ready to intervene with therapy if and when that conversion takes place. A recent study provides an example. In this study, 223 patients labeled as glaucoma suspects with no visual field defects were followed for an average of 63 months to assess conversion to glaucoma. Of these, 24% (54 eyes) converted to glaucoma with a mean time of 53 months.8
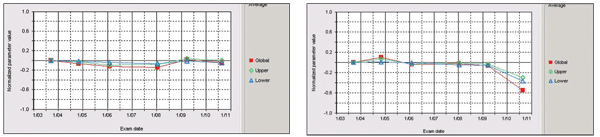
Plotting on HRT shows no progression in the left eye (at left). The right eye, however, does show progression.
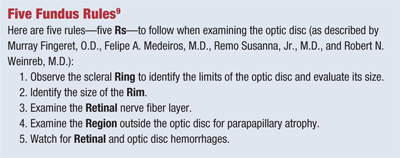
Monitoring entails regular IOP measurements and dilated fundus examination as well as completion of follow-up visual field and/or diagnostic laser imaging if available (see “Five Fundus Rules”).
Additionally, careful assessment of other risk factors, including history, needs to be performed. A reliable and repeatable change in the visual field and/or diagnostic laser imaging changes of a glaucomatous nature indicates conversion to glaucoma, which dictates a need to establish a target IOP and initiate treatment.
Diagnosis and Treatment
In cases where a visual field defect characteristic of glaucoma is identified (in the absence of other conditions that may produce such defects), the patient is diagnosed with glaucoma and a target IOP is set and treatment is initiated.
In cases where visual field defects are not identified but other suspicious findings (such as visible nerve fiber defects or very high IOP) and/or risk factors are noted, treatment may still be considered after input and discussion with the patient.
Usually, treatment is based on IOP readings combined with pachymetry and risk factors that indicate a likelihood of progression even without the presence of a visual field defect.
Additionally, an increase in the number of risk factors such as race, age and family history, along with a suspicious optic nerve appearance, will lower the clinician’s threshold for initiating treatment.
The target IOP is set at a level likely to be protective against glaucomatous optic nerve damage. While it is not known specifically at what IOP optic nerve damage will occur in a particular patient, many clinicians have traditionally set target IOP at 20% to 30% below the established baseline IOP measurements.
Bear in mind that the target IOP is an estimate and may change throughout the course of the disease, depending on stability of findings as well as the potential of new development of risk factors (e.g., trauma or systemic history complications).
In regard to the patient introduced at the beginning of this article: Where does he stand?
With repeated IOP measurements in the mid-20s, presence of a disc hemorrhage O.S. and significant risk factors for the development of glaucoma, we diagnosed this patient as a high-risk glaucoma suspect.
In conjunction with the patient’s wishes, we elected to start treatment with a prostaglandin analog and set a target pressure in the mid-teens. Treatment readily achieved the target pressure.
Glaucoma diagnosis and management is a long, ongoing process that incorporates risk factors and data collected from multiple exam elements and specialized testing. The clinician collects and analyzes the data and assesses the risk. A complete history and careful nerve head evaluation is paramount in beginning the process, along with the utilization of specialized testing.
Again, the ultimate goal is to preserve vision in a manner that allows patients to be active participants in their own care along with treatment that minimizes the negative effects on quality of life.
Dr. Black is an assistant professor and Chief of Primary Care Service at The Eye Care Institute at Nova Southeastern University College of Optometry. Dr. Tyler is an associate professor and Module Chief at Nova Southeastern.
1. Lee PP, Walt JG, Doyle JJ, Kotak SV, et al. A Multicenter, Retrospective Pilot Study of Resource Use and Costs Associated With Severity of Disease in Glaucoma. Arch Ophthalmol 2006;124:12-9.
2. Quigley HA, Addicks EM, Green WR. Optic Nerve Damage in Human Glaucoma III. Quantitative Correlation of Nerve Fiber Loss and Visual Field Defect in Glaucoma, Ischemic Neuropathy, Papilledema, and Toxic Neuropathy. Arch Ophthalmol 1982;100:135-46.
3. Large Optic Disc Nerve Heads: Megalopapilla or Megalodiscs. International Ophthalmology 2001;23:69-75.
4. Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic Evaluation of the Optic Nerve Head. Surv Ophthalmol 1999;43: 293-320.
5. Medeiros FA, Alencar LM, Sample PA, Zangwill LM, et al. The Relationship between Intraocular Pressure Reduction and Rates of Progressive Visual Field Loss in Eyes with Optic Disc Hemorrhage. Ophthalmology 2010;117:2061-6.
6. Brandt JD, Beiser JA, Kass MA, Gordon MO, et al. Central Corneal Thickness in the Ocular Hypertensive Treatment Study (OHTS). Ophthalmology 2001; 108:1779-88.
7. Huang JY, Pekmezci M, Mesiwala N, Koa A, et al. Diagnostic Power of Optic Disc Morphology, Peripapillary Retinal Nerve Fiber Layer Thickness, and Macular Inner Retinal Layer Thickness in Glaucoma Diagnosis With Fourier-domain Optical Coherence Tomography. J Glaucoma 2011;20:87-94.
8. Alencar LM, Bowd C, Weinreb RN, Zangwill LM, et al. Comparison of HRT-3 Glaucoma Probability Score and Subjective Stereophotograph Assessment for Predicition of Progression in Glaucoma. Invest Ophthalmol Vis Sci 2008;49:1898-1906.
9. Fingeret, M, Medeiros FA, Susanna R, Weinreb RN. Five Rules to Evaluate the Optic Disc and Retinal Nerve Fiber Layer for Glaucoma. Optometry. 2005;76:661-8.

