 A 74-year-old white male presented in January 2012 as a new patient. His primary care physician referred him to us for a complete ophthalmic evaluation, primarily because of a history of diabetes and post-cerebral aneurismal repair damage to the right eye.
A 74-year-old white male presented in January 2012 as a new patient. His primary care physician referred him to us for a complete ophthalmic evaluation, primarily because of a history of diabetes and post-cerebral aneurismal repair damage to the right eye.
Current medications included Synthroid (levothyroxine sodium, Abbott), Lopressor (metoprolol tartrate, Novartis), Diovan (valsartan, Novartis), Tricor (fenofibrate, Abbott), Niaspan (niacin, Abbott), pravastatin and metformin. He reported no drug allergies. The patient said that he has had type 2 diabetes mellitus for five years, and was started on diabetic medications only because of slightly abnormal glucose levels. That morning, his fasting blood glucose was 106mg/dl; he was unaware of his A1C level.
He underwent coronary artery stenting three years ago, as well as a cardiac ablation for arrhythmia several years prior to stent insertion.
The patient further reported that he underwent a procedure in 1999 to repair a cerebral aneurysm (procedure and specific site unknown); during the surgery, the right optic nerve was “deprived of oxygen,” he said, and he has since had a nasal field defect in the right eye. Other than this, he had no complaints related to his vision.
He had bilateral cataract surgery in 2006, and his last visit to an eye care provider was a follow-up to his cataract surgery.
Diagnostic Data
Entering visual acuity was 20/30-1 O.D. and 20/30-2 O.S. Pupils were round and reactive to light, with a +2 afferent defect (APD) noted O.D. Extraocular motilities were full in all positions of gaze. Best-corrected visual acuity was 20/30+1 O.D. and 20/20-1 O.S. through hyperopic astigmatic correction.
Slit lamp exam of his anterior segments was unremarkable. Intraocular pressure measured 17mm Hg O.D. and 18mm Hg O.S. at 9:58 a.m. Threshold visual field testing in the right eye revealed a dense scotoma above and below the midline, located primarily in the nasal field encroaching on superior fixation, but clearly not respecting the vertical midline. The visual field in the left eye was normal.
Dilated exam showed that the posterior chamber IOLs in both eyes were centered in the capsular bags. The right optic nerve was moderately pale in all quadrants, especially noticeable in the temporal aspect of the disc. His cup-to-disc ratio O.D. appeared to be 0.55 x 0.75, with thinning of the inferotemporal neuroretinal rim. The optic nerve of the left eye was characterized by a plush and well-perfused neuroretinal rim, and a cup-to-disc ratio of 0.40 x 0.40. Both nerves were of average size.
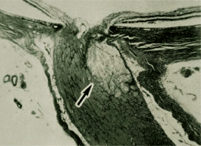
This micrograph shows an ischemic optic neuropathy, similar to what we suspect in this patient's case. The arrow points to the area of infarct--where the optic nerve is supplied by the short posterior ciliary arteries.
The retinal vasculature was characterized by mild hypertensive and arteriolar sclerotic retinopathy O.U., consistent with his medical history. The right macula had a small, diffuse epiretinal membrane involving the foveal avascular zone, along with fine retinal pigment epithelial (RPE) granulation consistent with his age. The left macula was remarkable only for symmetric RPE granulation. There were no findings in either eye associated with his non-proliferative diabetic retinopathy.
His peripheral retinal examination was unremarkable O.U. We obtained fundus photos as well as B-scan ultrasonography O.U., primarily aimed at evaluating the physical characteristics of the post-laminar anterior optic nerves. Optic nerves in both scans were symmetric and normal.
Now, how do you proceed? When do you see him back, and for what?
Discussion
Although this patient has a complex history, there is no evidence from the preliminary visit that there is any imminent threat to his vision. Certainly, there are unanswered questions—foremost of which is the status of the right optic nerve. He presented with a visual field defect and an APD in the same eye, but his history suggests that these findings coincided with each other as complications related to his cerebral aneurysm repair. And, most importantly, his history indicated that the visual field defects in the right eye were stable.
But, are they really stable? Also, was there anything in the examination that suggests possible progressive visual field loss? The answer to this question drives the scheduled follow-up visits.
Optic nerve ischemia occurs after many types of surgery. Whether through blood loss, extended operative time, associated cardiovascular and pulmonary co-morbidities or trauma, the optic nerve depends on proper oxygenation for normal functioning. Abnormalities in this process are the basis of the non-glaucomatous optic neuropathies.
But the non-glaucomatous optic neuropathies result in optic nerve damage fundamentally different than that of glaucomatous optic neuropathy.
In cases of non-glaucomatous optic neuropathies, the visible end result of the optic nerve damage is typically optic disc pallor and optic atrophy. But, in such cases, the neuroretinal rims typically do not become thinned. While they become pale and atrophic over time, the ganglion cells of the neuroretinal rim usually remain in place.
In glaucomatous optic neuropathy, however, the rim tissue remains pink and perfused, but there is characteristic loss of neuroretinal rim and nerve fibers.
The key to this patient’s future visits, at least in the immediate future, relate to the fact that his right optic nerve is characterized by both neuroretinal rim loss consistent with glaucoma and optic nerve pallor. While it’s very likely that the optic disc pallor indicates a posterior ischemic optic neuropathy, which probably occurred as a result of the intracranial aneurysm repair, we still need to account for the etiology of the neuroretinal rim loss.
We scheduled the patient for further evaluation three weeks after his initial presentation. This will include color vision tests, Heidelberg Edge Perimeter visual fields, corneal pachymetry, gonioscopy, Heidelberg Retina Tomograph-3 optic nerve imaging and stereo-optic nerve imaging.
Does this patient have two co-existing optic nerve morbidities? Possibly. When will we find out? Very soon.

