Although glaucoma is the most prevalent optic neuropathy seen by clinicians, others can be mistaken for it, or vice-versa, especially in late-stage cases. Glaucoma’s defining characteristics, such as optic disc cupping and visual field loss, are shared with progressive stages of anterior ischemic optic neuropathy (AION), including an excavated, atrophic and pale optic nerve and similar visual field defects. For this reason, a team of Belgian and Portuguese researchers recently conducted a systematic literature review to summarize differences in diagnostic tests that can help perform a correct diagnosis of AION, and came away noting the value of certain OCT imaging parameters.
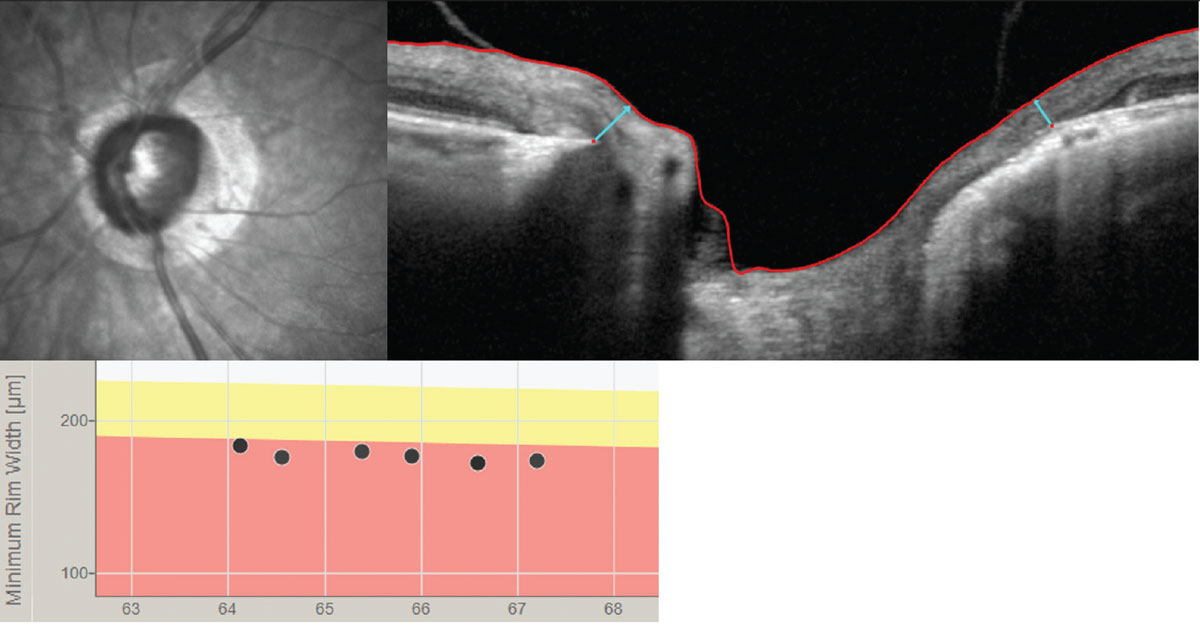 |
| Comparing certain OCT imaging markers such as Bruch’s membrane opening–minimum rim width parameters may help clinicians distinguish between glaucoma and anterior ischemic optic neuropathy. Photo: Andrew Rixon, OD. Click image to enlarge. |
The paper, which included a total of 3,307 eyes from 32 studies, noted clear differences in the OCT findings on optic nerve head and retinal nerve fiber layer (RNFL) parameters for the two diseases. Glaucomatous eyes had a greater cup area, cup-to-disc ratio, cup volume and cup depth, and a lower (vertical) rim volume and rim area compared to AION eyes. The study authors said Bruch's membrane opening should also be considered. “Several findings indicate that nonarteritic AION eyes had a thicker Bruch’s membrane opening–minimum rim width (BMO-MRW) in contrast to glaucomatous eyes who showed a greater thinning of the BMO-MRW,” they said. “The lowest MRW values could be found in the temporal and inferotemporal sectors in glaucomatous eyes. Consequently, the MRW/RNFL ratios were higher in NAION eyes than in glaucomatous or healthy eyes.” Furthermore, thinning and/or retro-displacement of the supporting connective tissues of the lamina was more pronounced in open-angle glaucoma than AION. Most of the OCT findings in the study indicated that RNFL thinning in glaucomatous eyes occurred most commonly at the superotemporal and inferotemporal regions, whereas thinning in nonarteritic AION showed at the superonasal region.
In the macula, significantly more ganglion cell–inner plexiform layer thinning was observed in NAION compared to glaucomatous eyes, except in the inferotemporal sector where slightly more thinning was found in glaucomatous eyes, although not significant. There was also a significant decrease in superficial macular vessel density in glaucoma compared to AION with a similar degree of visual field deficit.
The authors stated that selection bias can’t be eliminated, due to the review’s restriction to the English literature, and also noted that “several factors were not considered when comparing the results, including the distinction between normal tension glaucoma and other types of glaucoma, as well as the possible influence of systemic medication” use such as glaucoma meds and/or antihypertensives. Lastly, they commented on how heterogeneity in study design can influence results. “Not all studies accounted for the difference in disease severity when comparing AION to glaucoma. In an ideal setup, all studies would have a visual field (or mean-deviation) matched comparison to more accurately compare both disease entities.”
That said, this review highlights the value of OCT as an additive tool in identifying optic neuropathies, while also reiterating the importance of clinical examination to distinguish glaucomatous optic neuropathies vs. non-glaucomatous forms.
Smeets F, Margot A, Barbosa-Breda J, Stalmans I, Lemmens S. Differentiating ischemic optic neuropathy from glaucoma using diagnostic tests. Ophthalmic Res. January 23, 2024. [Epub ahead of print]. |

