 Punctal plugs have been around since the 1970s. But, the concept of punctal occlusion, blockading the lacrimal drainage system to enhance contact time between the tears and ocular surface, was first described in 1935.1 Forty years later, Jerre Freeman, M.D., developed the first punctal plug, composed of silicone and designed to be removable should patients experience adverse effects.2
Punctal plugs have been around since the 1970s. But, the concept of punctal occlusion, blockading the lacrimal drainage system to enhance contact time between the tears and ocular surface, was first described in 1935.1 Forty years later, Jerre Freeman, M.D., developed the first punctal plug, composed of silicone and designed to be removable should patients experience adverse effects.2
The insertion of punctal plugs is relatively simple and often quite effective. At least ten different manufacturers produce and market more than 25 different types and styles of plugs in the
Despite their great popularity and longevity, the clinical benefit of punctal plugs has been called into question. In 1998, one study suggested that, while plugs offer improvement of symptoms and signs of dryness There seems to be some decrease in improvement of symptoms with the plugs over time.3
Likewise, one study of punctal occlusion in normal subjects found that tear production decreased after temporary punctal occlusion There appears to be an autoregulatory mechanism to return tear production, tear clearance and ocular surface sensation to pre-occlusion levels 14 to 17 days after punctal occlusion.4
Do these studies explain why many patients respond positively to punctal plugs immediately following the procedure, but ultimately experience a recurrence of their dry eye symptoms?
The Evidence
Two pivotal reports on dry eye disease discuss the appropriate use of punctal plugs. The Dysfunctional Tear Syndrome Study Group (the Delphi panel) concluded that punctal occlusion was most appropriate for patients who had moderate to severe dry eye disease and did not adequately respond to lubrication therapy and topical immunomodulators, such as Restasis (cyclosporine, Allergan).5 Their rationale for not recommending plugs in earlier stages of the disease was that punctal plugs could result in retention of pro-inflammatory tear components on the ocular surface and may enhance damage to the ocular surface, accelerate the disease process, and produce greater patient discomfort, and that it is important to treat the inflammatory condition before blockage of tear drainage with punctal plugs.5
These conclusions were echoed in the 2007 Report of the International Dry Eye Workshop (DEWS). This group agreed that management of ocular surface inflammation is beneficial prior to punctal occlusion; however, based on a treatment algorithm published in the report, DEWS suggested that punctal plugs may be beneficial in somewhat earlier stages of dry eye, perhaps even concurrently with topical immunomodulatory therapy.6
The original trials of cyclosporine found that increased tear production did not occur in patients using punctal plugs; but, a recent study refutes these conclusions.7,8 In this 2007 study, patients using punctal plugs and topical cyclosporine together showed greater overall improvement than with plugs or cyclosporine therapy alone.8 The authors concluded that a possible additive effect of topical cyclosporine and punctal occlusion might merit concomitant use.8
The most common indication for punctal occlusion is dry eye, but many other conditions may benefit from such treatment.9 For example, the ocular surface disorders filamentary keratitis, superior limbic keratoconjunctivitis, trachoma and neurotrophic keratopathy have shown improvement with punctal plugs.10-13
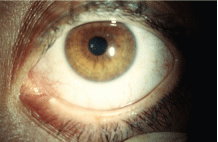
Occlusion of the lower puncta with silicone plugs is a common treatment for moderate to advanced dry eye.
The use of punctal occlusion with plugs might also be advantageous in patients on topical glaucoma therapy, since increasing the contact time between medication and cornea could enhance ocular penetration and efficacy.14 But, prospective clinical studies have failed to demonstrate significant intraocular pressure differences between glaucoma patients who use plugs and those who do not.14,15
Perhaps the greatest emerging use for punctal plugs within the last five to 10 years is in the management of post-refractive surgery complications. The use of dissolvable punctal plugs for at-risk patients undergoing LASIK or similar procedures is the standard of care for some surgical practices. And, research has demonstrated that intensive management of the ocular surface with punctal plugs after photorefractive keratectomy (PRK) or LASIK can diminish symptoms, improve conjunctival goblet cell density, enhance visual acuity and reduce wavefront aberrations.16-18
Further Questioning
Though typically safe, punctal occlusion is not without potential for complications. A prospective study found that more than 44% of eyes treated experienced loss of plugs within two years.19 Other complications can be more serious; irritation, infection, and chronic inflammation (e.g., canaliculitis or granuloma formation) have been reported with a variety of plugs.19-21 In particular, the Medennium SmartPLUG, an intracanalicular thermoacrylic occlusion device, has been associated with inflammatory complications of the nasolacrimal system.21-23
Despite the potential controversies, the incidence with which practitioners utilize punctal occlusion has shown no signs of diminution.24 Data from the Centers for Medicare and Medicaid Services indicate that this procedure has actually grown in popularity over the past five years.24
At present, punctal occlusion is the single most popular minor procedure in optometry, with about 98,000 plugs inserted per year.24 Within ophthalmology, the incidence is even higher, with about 203,000 annual procedures.24 This includes non-dissolvable silicone plugs, collagen (which dissolves over several days to a week) and extended-duration polymers (which dissolve over several months).
Punctal occlusion remains an importantthough somewhat controversialtherapeutic option for patients with ocular surface disease. Understanding the risks, benefits and potential limitations of this procedure helps us to utilize it more effectively in the care of our patients.
Drs. Kabat and Sowka have no direct financial interest in any of the companies or products mentioned in this article. They thank Dr. John Rumpakis for his help in researching some of the material discussed herein.
1. Beetham WP. Filamentary keratitis. Trans Am Ophthalmol Soc 1935;33:413-35.
2. Freeman JM. The punctum plug: evaluation of a new treatment for the dry eye. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1975;79(6):OP874-9.
3. Slusser TG, Lowther GE. Effects of lacrimal drainage occlusion with nondissolvable intracanalicular plugs on hydrogel contact lens wear. Optom Vis Sci 1998 May;75(5):330-8.
4. Yen MT,
5. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a
6. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007 Apr;5(2):163-78.
7. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000 Apr;107(4):631-9.
8. Roberts CW,
9. Baxter SA, Laibson PR. Punctal plugs in the management of dry eyes. Ocul Surf 2004 Oct;2(4):255-65.
10. Albietz J, Sanfilippo P, Troutbeck R, Lenton LM. Management of filamentary keratitis associated with aqueous-deficient dry eye. Optom Vis Sci 2003 Jun;80(6):420-30.
11. Kabat AG. Lacrimal occlusion therapy for the treatment of superior limbic keratoconjunctivitis. Optom Vis Sci 1998 Oct;75(10):714-8.
12. Guzey M, Ozardali I, Kilic A, et al. The treatment of severe trachomatous dry eye with canalicular silicone plugs. Eye 2001 Jun;15(Pt 3):297-303.
13. Tai MC, Cosar CB, Cohen EJ, et al. The clinical efficacy of silicone punctal plug therapy. Cornea 2002 Mar;21(2):135-9.
14. Bartlett JD, Boan K, Corliss D, Gaddie IB. Efficacy of silicone punctal plugs as adjuncts to topical pharmacotherapy of glaucoma--a pilot study. Punctal Plugs in Glaucoma Study Group. J Am Optom Assoc 1996 Nov;67(11):664-8.
15. Aritrk N, Oge I, Erkan D, et al. The effects of nasolacrimal canal blockage on topical medications for glaucoma. Acta Ophthalmol Scand 1996 Aug;74(4):411-3.
16. Albietz JM, McLennan SG, Lenton LM. Ocular surface management of photorefractive keratectomy and laser in situ keratomileusis. J Refract Surg 2003 Nov-Dec;19(6):636-44.
17. Huang B, Mirza MA, Qazi MA, Pepose JS. The effect of punctal occlusion on wavefront aberrations in dry eye patients after laser in situ keratomileusis. Am J Ophthalmol 2004 Jan;137(1):52-61.
18. Toda I. LASIK and dry eye. Compr Ophthalmol Update 2007 Mar-Apr;8(2):79-85; discussion 87-9.
19. Horwath-Winter J, Thaci A, Gruber A, Boldin I. Long-term retention rates and complications of silicone punctal plugs in dry eye. Am J Ophthalmol 2007 Sep;144(3):441-4.
20. Toufeeq A, Mohammad-Ali FH. Peripheral corneal ulceration as a complication of silicon punctal plug: a case report. Eye 2007 Nov;21(11):1437-8.
21. Chou TY, Perry HD, Donnenfeld ED, Solomon R. Pyogenic granuloma formation following placement of the Medennium SmartPLUG punctum plug. Cornea 2006 May;25(4):493-5.
22. Scheepers M, Pearson A, Michaelides M. Bilateral canaliculitis following SmartPLUG insertion for dry eye syndrome post LASIK surgery. Graefes Arch Clin Exp Ophthalmol 2007 Jun;245(6):895-7.
23. SmartPlug Study Group. Management of complications after insertion of the SmartPlug punctal plug: a study of 28 patients. Ophthalmology 2006 Oct;113(10):1859.e1-6.
24. Centers for Medicare and Medicaid Services. Part B Extract Summary System (BESS) Data File. CD-ROM. Available at: www.cms.hhs.gov/NonIdentifiableDataFiles/03_PartBExtractSummarySystem.asp#TopOfPage. (Accessed 2006).

