Pupil testing can reveal serious retinal and neuro-ophthalmic disease and therefore should be incorporated into every comprehensive eye examination. With careful clinical examination, this test can aid in the diagnosis and management of many of these conditions at the primary care level. This article addresses the more commonly encountered pupil disorders and how clinicians can detect them through routine pupil testing.
Neuroanatomy
Meaningful interpretation of pupillary findings requires a solid working knowledge of the anatomy of the light reflex and the autonomic innervation of pupillary responses. The pupillary light and near responses are under parasympathetic innervation. The pupillary light response consists of both an afferent and efferent pathway. The afferent pathway is responsible for transmitting the impulse of the incoming light via the photoreceptors of the retina, through the optic nerve to the chiasm and optic tract, then separate from the tract just anteriorly to the lateral geniculate body (LGN) before traveling to the mid-brain to bilaterally project to the pretectal nuclei.1 Pupil fibers synapse in the pretectal nuclei of the midbrain and travel to the two Edinger-Westphal nuclei of the oculomotor nerve (CN III), beginning the efferent pathway.1
 |
| Fig. 1. Neutral density filters can be useful in grading relative afferent pupillary defects. |
Because of this neuroanatomy, we are able to objectively measure the integrity of the afferent pathway by observing the direct and consensual light responses. For example, the direct response of the right eye (and consensual response of the left eye) indicates the integrity of the afferent pathway on the right side. This is also the reason why a lesion of the optic nerve or optic tract does not result in anisocoria, or difference in pupil size between the two eyes. Efferent pupil fibers then travel with CN III back towards the orbit, where they synapse in the ciliary ganglion, with 3% of post-ganglionic fibers innervating the iris sphincter muscle (which allows for miosis) and the remaining 97% innervating the ciliary body (which allows for accommodation).1
Oculo-sympathetic innervation to the eye consists of a three-neuron arc. Originating in the posterior hypothalamus, the first-order neuron descends through the brainstem to synapse in the ciliospinal center of Budge between the levels of the eighth cervical and fourth thoracic vertebrae (C8-T4). The second-order neuron leaves the spinal cord and passes over the apex of the lung to synapse at the superior cervical ganglion. Third-order neurons give rise to post-ganglionic axons, which leave the superior cervical ganglion and run along the course of the internal carotid artery through the cavernous sinus, where they meet up with the ophthalmic division of the trigeminal nerve (V1) and ophthalmic artery to travel to the eye. Neurons traveling with the ophthalmic artery go on to innervate Mueller’s muscle for eyelid control, whereas those traveling with V1 pass through the ciliary ganglion to innervate the iris dilator muscle, which allows for mydriasis.2,3
Pharmacologic Testing for Horner’s Syndrome15 | ||||
| Location of Lesion | For Diagnosis | For Localization | ||
| Cocaine 10% | Apraclonidine 0.5%-1% | Hydroxyamphetamine 1% | Phenylephrine 1% | |
| Normal pupil (no lesion) | Dilates | Will not dilate | Dilates | Will not dilate |
| First or second order (pre-ganglionic) | Will not dilate | Dilates pupil (and reverses anisocoria) | Dilates | |
| Third order (post-ganglionic) | Will not dilate | Dilates | ||
The Swinging Flashlight Test
Clinicians use the swinging flashlight test to detect an afferent pupillary defect and should conduct the test in a dark room with a transilluminator or the light from the binocular indirect ophthalmoscope, which are preferred over a handheld penlight due to the intensity of the light. The strength of the direct pupillary response is compared with that of the consensual pupillary response in the same eye. When the consensual response is greater than the direct response in the affected eye, the patient has a relative afferent pupillary defect (RAPD), also known as an APD or Marcus Gunn pupil, signifying damage at or anterior to the LGN.4 To cause an RAPD, the damage must be unilateral or asymmetric, such as in severe retinal disease, optic nerve disease or compromise, or a lesion behind the eye. Severe but bilaterally equal disease will not result in an RAPD, as a bilateral APD does not exist. In addition, an RAPD cannot be caused by disorders of ocular media or refraction, even if extreme.4 Visual acuity does not necessarily correlate with an RAPD; however, clinicians should always look carefully for one in cases of significantly reduced acuity in one eye.
RAPDs can also be assigned a grade using a neutral density filter over the good eye to quantify the defect. Neutral density filters are available in a variety of densities, with 0.3, 0.6, 0.9 and 1.2 log units being most helpful in grading an RAPD (Figure 1). Grading can be helpful for identifying subtle defects and monitoring for progression. Newer, high-definition technology is available for pupil diagnostics, allowing for objective, detailed and quantitative measurements of both the direct and consensual light responses.5
| Pharmacologic Testing for the Dilated Pupil using Pilocarpine | ||
| Finding | Pilocarpine 0.125% | Pilocarpine 1% |
| Normal pupil | Does not constrict | Constricts |
| Adie’s pupil | Constricts | Constricts |
| CN III palsy | Usually does not constrict | Constricts |
| Pharmacologic dilation | Does not constrict | Does not constrict |
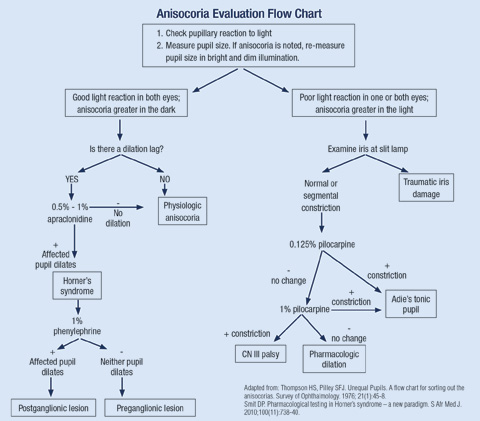
Because of the consensual response, only one functioning pupil is needed to test for an RAPD in either eye. When testing only one pupil, the swinging flashlight test is performed in the same way as you would with two functioning pupils; however, only the reactive pupil is observed. The direct and consensual responses are compared in the reactive pupil, and if the pupil constricts more with direct illumination than with consensual, then the RAPD is present in the opposite eye with the unreactive pupil. If the reactive pupil constricts more with consensual stimulation than with direct illumination, the RAPD exists in the eye with a reactive pupil. This is known as determining an APD by reverse.
Evaluating Pupil Shape and Size
Pupils should be round, symmetrical and centered within the iris. Pupil size is influenced by smooth muscles under control of the autonomic nervous system, with the iris sphincter (parasympathetic innervation) having more powerful control than the iris dilator (sympathetic innervation) in maintaining pupil size and controlling the amount of light that enters the eye.6
Due to changes in lighting and accommodation, a patient’s pupil size is constantly fluctuating. One of the initial elements of pupil testing should be to measure the patient’s pupil diameter to look for any evidence of anisocoria. Under normal illumination, the average adult’s pupil size measures around 3.5mm, but can vary from 1.0mm to 10mm and get smaller as one ages due to senile miosis.7 A difference of 0.4mm or greater between the two eyes is considered clinically significant.8 If a patient has anisocoria, typically only one pupil is abnormal, and the etiology can be either physiologic (which occurs in approximately 20% of normal patients), pharmacologic or pathologic in nature.9,10
Any anisocoria should be further evaluated by re-assessing pupil size in both bright and dark illumination to help isolate the parasympathetic and sympathetic pathways, respectively. If the difference between pupil sizes becomes greater in the bright illumination, the larger pupil is the abnormal one, indicating parasympathetic denervation. If the difference becomes greater in dark illumination, the smaller pupil is the abnormal one due to abnormal sympathetic innervation.
In either situation, a thorough and thoughtful history should accompany any suspected pupil anomaly, which may provide clues to the appropriate diagnosis, such as whether the patient recently took or came into contact with medications or agents that can affect pupil size, or they have a history of recent trauma or surgery. Clinicians can also look at old photos or a patient’s driver’s license to better understand the possible onset or duration.
If the anisocoria is physiologic in nature, the difference in pupil size between the two eyes should remain constant in all lighting conditions. Physiologic anisocoria is seldom greater than 1mm, can be variable from day to day and can even switch eyes.9,10 No further pharmacologic evaluation is necessary if physiologic anisocoria is suspected, though clinicians should always rule out other neuropathologies.
Pharmacological Pupil Testing
In the presence of a normal pupillary response, pharmacological pupil testing can help differentiate the various causes of anisocoria. First, the clinician will need to determine which pupil is the problematic pupil.
Small Pupil Problems
If the anisocoria is greater in the dark, practitioners should focus on the smaller pupil as the abnormal pupil and investigate impairment of the oculo-sympathetic system. Common causes include:
• Pharmacologic constriction. If this is due to systemic drugs such as morphine, heroin or codeine, the miosis is typically bilateral. If the constriction is unilateral, the patient may have used a cholinergic agonist such as pilocarpine or had contact with a specific anticholinesterase agent, such as a flea/tick control product, which can cause a heightened parasympathetic effect.
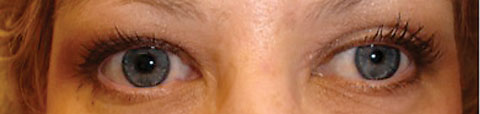 |
| Fig. 2. Horner’s syndrome OS with ipsilateral miosis and ptosis. |
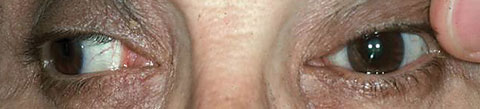
• Horner’s syndrome. Also known as oculo-sympathetic paresis, Horner’s syndrome represents an interruption somewhere along the long oculo-sympathetic nerve pathway between its origin in the hypothalamus and the eye. The classic triad of symptoms includes unilateral ptosis, ipsilateral miosis and facial anhidrosis—although these findings can be variable in presentation (Figure 2).11 One-third of cases are idiopathic, with no evident underlying cause.4 Another 4% to 13% are of congenital etiology and are characterized by iris heterochromia, with the lighter iris being the affected eye.4,12
Congenital cases result from trauma sustained during delivery or an idiopathic cause occurring before age two.12 Common etiologies of acquired preganglionic Horner’s syndrome include, but are not limited to: stroke, trauma, surgery, aortic or carotid artery dissection, Pancoast tumor and tuberculosis. Postganglionic acquired causes include trauma, painful cluster migraine headaches (Raeder’s syndrome), giant cell arteritis and neck/thyroid surgery.2 A detailed history and diagnostic imaging can help to differentiate between some of the causes of acquired Horner’s syndrome.
 |
| Click image to enlarge. |
Looking for evidence of a “dilation lag” in suspected Horner’s syndrome can be helpful, as the Horner’s pupil will be delayed in its dilation in a dark room. The anisocoria will be most evident about four to five seconds after the lights are turned off, then the abnormal pupil will slowly begin to dilate over the next 10 to 15 seconds, making the anisocoria less evident the longer the patient remains in the dark room.13,14 This dilation lag is a classic diagnostic sign of Horner’s syndrome and occurs secondary to passive dilation from relaxation of the iris sphincter in the Horner’s pupil as opposed to rapid, active dilation of a pupil with intact sympathetic function and a working dilator muscle.14 Pharmacologic testing will help confirm the diagnosis and further help localize the lesion to narrow down the differentials.
Topical cocaine 4% to 10% was initially used to confirm the diagnosis of Horner’s syndrome, as it blocks reuptake of norepinephrine at the nerve ending. The excess norepinephrine will cause a normal pupil to dilate, but a Horner’s pupil will fail to dilate because of the absence of norepinephrine at the receptor site.2-4,15 However, cocaine is difficult to obtain due to its scheduling status and has potential adverse side effects to the central nervous system. Recent literature has discussed a more favorable approach to confirming Horner’s syndrome using Iopidine (apraclonidine, Alcon).15,16 Iopidine is a readily available alpha-adrenergic receptor agonist typically used for its short-acting IOP lowering properties. Instilling one drop of 0.5% or 1% Iopidine results in dilation of a Horner’s pupil and the reversal of the anisocoria with the miotic pupil becoming larger than the normal pupil.15,16 Iopidine has little to no effect on a normal pupil.15,16 Clinically, dilation of 1mm or more is needed to confirm the presence of Horner’s.16 Clinicians should measure pupil size approximately 30 to 45 minutes after drop instillation.
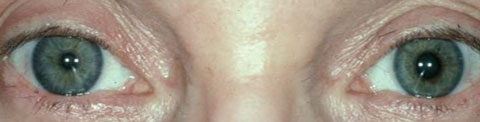 |
| Fig 3. Above, the patient has anisocoria with a suspected left tonic pupil. Below, post-pharmacologic testing with 0.125% pilocarpine shows constriction OS, demonstrating denervation supersensitivity. Photos: Mark Swanson, OD. |
 |
Following confirmation of the diagnosis of Horner’s syndrome, the next clinical step is to differentiate the lesion’s location. Twenty-four to 48 hours following testing with Iopidine or topical cocaine, practitioners can use pharmacologic testing with two drops of 1% Paredrine (hydroxyamphetamine, Akorn) to identify postganglionic (superior cervical ganglion to pupil) lesions from central or preganglionic lesions. Paredrine dilates a first- or second-order neuron lesion, as well as normal pupils, by releasing stored norepinephrine from the postganglionic axon terminals into the neuromuscular junction at the iris dilator.15 There is no pharmacological test to differentiate between a first- and second-order neuron lesion. In a Horner’s syndrome secondary to a postganglionic lesion, these fibers are damaged and the Paredrine fails to dilate the pupil, causing the degree of anisocoria to remain the same.
Because Paredrine is also difficult to obtain, phenylephrine 1% is often available, which can be diluted if needed from 2.5% solution, and should dilate a postganglionic Horner’s syndrome due to denervation super-sensitivity but not those due to central or preganglionic causes.17 Denervation super-sensitivity takes time to develop, so in acute onset cases, this may not hold true. Although it is recommended that clinicians wait 24 to 48 hours between pharmacological tests, there may be situations in which stat imaging would take precedence over attempting to localize a lesion. The patient’s history—whether the Horner’s is isolated or if there are associated findings such as diplopia, cranial nerve palsy, numbness, headache or pain—can help guide the management approach. Prompt imaging would be indicated in situations that point to a life-threatening etiology of Horner’s.
• Other oculo-sympathetic innervation problems. Sympathetic spasms are rare and can involve the entire pupil (intermittent mydriasis) or any segment of the pupil (tadpole-, or keyhole-shaped pupil). Many of these cases are benign in nature and occur most commonly in young females.18 Several reported cases have been later found to develop ipsilateral Horner’s syndrome, and the initial spasm is attributed to the firing of “sick neurons.”18 Thus, clinicians should test for Horner’s in these cases.18
• Argyll-Robertson pupils. Bilaterally small and irregular pupils with a near response markedly better than the light response (also known as “light-near dissociation”) are known as Argyll-Robertson pupils.4 Although the miosis is bilateral, it is often asymmetric, and these pupils are typically very difficult to dilate, which can help confirm the diagnosis.15 Because of the association with chronic syphilis, systemic laboratory testing, including FTA-Abs and VDRL, should be included in these patients’ workup.
Big Pupil Problems
If the anisocoria is greater in bright illumination, clinicians should focus on the larger pupil and investigate for parasympathetic denervation. Common causes include:
• Pharmacologic dilation. Many pharmacologic agents can result in pupillary dilation, and a careful history is needed to help rule out these causes. Anticholinergics that can result in dilation include agents such as scopolamine found in motion sickness patches or permethrin found in insecticides. Contact with various plant species such as angel’s trumpet, jimson weed and belladonna can also result in pupil dilation.19 Many OTC products containing phenylephrine—including antihistamines, redness relief drops and anti-itch creams—may also be common culprits resulting in pharmacologic dilation. Pharmacologically dilated pupils will not constrict with 1% pilocarpine.
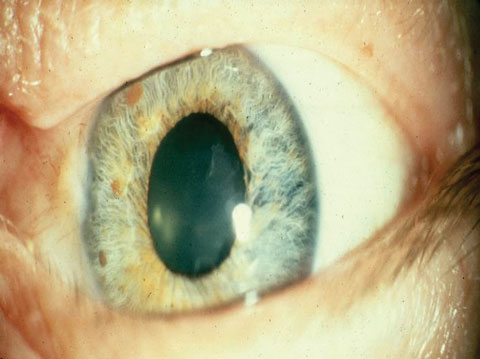
• Tonic pupils. Lesions of the ciliary ganglion or short posterior ciliary nerves within the orbit will produce a tonic pupil, characterized by findings such as segmental iris paralysis, light-near dissociation, tonicity to light and accommodation responses and denervation hypersensitivity to dilute cholinergic agents such as pilocarpine.1,4,20 Causes of a tonic pupil can include orbital trauma, viral illness, diabetes and syphilis.1,4 In older patients, clinicians should obtain an erythrocyte sedimentation rate (ESR) to rule out giant cell arteritis. When the tonic pupil is idiopathic, which is most often the case in 20- to 40-year-old females, the term Adie’s tonic pupil is used.1,4 Testing of deep tendon reflexes in the knee and ankle is often helpful to diagnose Adie’s syndrome, in which these reflexes are markedly diminished or absent.1,20
Due to denervation hypersensitivity of the iris sphincter, tonic pupils will constrict with a weak concentration of 0.125% pilocarpine, whereas this concentration is ineffective in normal pupils (Figure 3).1,4,15 Commercially available pilocarpine can be diluted in office using seven drops of saline to one drop of 1% pilocarpine, or 15 drops of saline to one drop of 2% pilocarpine. Clinicians can mix the agents in a contact lens case, using a syringe to ensure equal saline and pilocarpine drop sizes when mixing.
 |
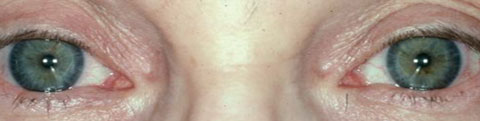
| Fig. 4. Segmental iris paralysis noted in tonic pupils, best observed under slit lamp magnification. Photo: Mark Swanson, OD. |
Frequently, tonic pupils are diagnosed behind the slit lamp. Clinicians can use the biomicroscope to look for segmental pupillary sphincter palsies or segmental constriction found in tonic pupils to assist in the diagnosis (Figure 4). Turning the rheostat on and off while the light beam is placed at the pupil margin can be helpful to look for these characteristic findings.
There is no definitive treatment for patients with tonic pupils. Mild miotics such as brimonidine, low-dose pilocarpine or specialty iris-simulating contact lenses may be helpful for patients symptomatic for glare caused by the mydriasis.1,4 The tonic pupil is usually unilateral, but can become bilateral at a rate of approximately 4% per year.1 In addition, the amount of anisocoria tends to gradually diminish, as the larger tonic pupil becomes more miotic over time.1,4
• Cranial nerve III palsy. The typical presentation in an isolated CN III palsy is ptosis along with exotropia and hypotropia causing the eye to be in a “down and out” position (Figure 5). Because the pupillary fibers are located close to the surface of CN III, they are more susceptible to compression via a mass or aneurysm and are more likely to result in a pupil-involving CN III palsy.4
 |
| Fig. 5. Pupil involving CN III palsy in the left eye. Photo: Mark Swanson, OD. |
In all cases of pupillary involvement, immediate neurosurgical consult with neuroimaging and angiography is indicated. Ensure this occurs by sending the patient to the emergency department immediately and notify the hospital in advance of the incoming patient with the potential for a life-threatening condition.
An aneurysm of the posterior communicating artery presents with a CN III palsy 30% to 60% of the time.23 In addition, other causes such as tumors and trauma must also be ruled out in pupil-involving CN III palsies.23 Although pupil-sparing CN III palsies tend to be ischemic in nature, this rule is not absolute, as pupil-sparing may become pupil-involving over time.21 Up to 14% of CN III palsies due to aneurysm may not show pupil involvement in the early stages.23 Careful and close follow up is indicated for all patients in which the pupil is spared—especially if there is no underlying systemic diabetes or hypertension.
Visual acuity tends to be unaffected in these patients, and the pupil will constrict with 1% pilocarpine. Clinicians should always consider consulting with a neurologist, even when they suspect a CN III palsy not caused by an aneurysm.
Careful observation of the pupils can reveal important information about the autonomic nervous system, and further evaluation with pharmacologic testing can help confirm sympathetic or parasympathetic deficits. These clinical observations and tests can ensure ODs provide patients and comanaging practitioners the information necessary for appropriate treatment, even for those who may not even know they have a neurological condition.
Dr. Pate is an associate professor at the UAB School of Optometry in Birmingham, AL.
|
1. Gurwood AS, Lehn LE. Tonic Pupils. Optometry Today. 1999;39(13):37-9. 2. Patel S, Ilsen PF. Aquired Horner’s syndrome: clinical review. Optometry. 2003;74(4):245-56. 3. Gurwood AS. Horner’s Syndrome. Optometry Today. 1999;39(11):36-7. 4. Wilhelm H. Neuro-ophthalmology of pupillary function – practical guidelines. J Neurol. 1998;245(9):573-83. 5. Konan Medical USA. RAPDx. Accessed 18 Jan 2016. http://konanmedical.com/rapdx/. 6. Purves D, Augustine GJ, Fitzpatrck D, et al. Neuroscience. 4th ed. Sunderland, MA: Sinauer Associates; 2008:290–1. 7. Pensyl CD, Benjamin WJ. Ocular Motility. Borish’s Clinical Refraction 2nd ed. St. Louis: Butterworth Heinemann Elsevier; 2006:356-65. 8. Loewenfeld IE. Simple, central anisocoria: A common condition seldom recognized. Trans Am Acad Ophthalmol Otolaryngol. 1977;83:832. 9. Lam BL, Thompson HS, Corbett JJ. The prevalence of simple anisocoria. Am J Ophthalmol. 1987 Jul 15;104(1):69-73. 10. Rosenberg ML. Physiologic anisocoria: A manifestation of physiologic sympathetic asymmetry. Neuro-Ophthalmology. 2008;32:147-9. 11. Walton K. Buono L. Horner syndrome. Curr Opin Ophthal. 2003;14;357-63. 12. Bell RL, Atweh N, Ivy ME, Possenti P. Traumatic and iatrogenic Horner syndrome: case reports and review of the literature. J Trauma. 2001;51(2):400-4. 13. Bremner F. Pupil evaluation as a test for autonomic disorders. Clin Auton Res. 2009;19:88-101. 14. Crippa SV, Borruat FX, Kawasaki A. Pupillary dilation lab is intermittently present in patients with a stable oculosympathetic defect (Horner Syndrome). Am J Ophthalmol. 2007 Apr;143(4):712-5. 15. Bennetto L, Guly C, Ormerod I, Plant GT. Eye drop neurology. Pract Neurol. 2014;14:145-51. 16. Smit DP. Pharmacological testing in Horner’s syndrome – a new paradigm. S Afr Med J. 2010;100(11):738-40. 17. Danesh-Meyer HV, Savino P, Sergott R. The correlation of phenylephrine 1% with hydroxyamphetamine 1% in Horner’s syndrome. Br J Ophthalmol. 2004;88:592-3. 18. Balaggan KS, Hugkulstone CE, Bremner FD. Episodic segmental iris dilator muscle spasm: The tadpole-shaped pupil. Arch Ophthalmol. 2003;121:744. 19. Vunda A, Alcoba G. Mydriasis in the garden. New Eng J Med. 2012;367:1341. 20. Leavitt JA, Wayman LL, Hodge DO, Brubaker RF. Pupillary response to four concentrations of pilocarpine in normal subjects: application to testing for Adie tonic pupil. Am J Ophthalmol. 2002;133(3):333-6. 21. Kissell JT, Burde RM, Klingele TG, Zeiger HE. Pupil-sparing oculomotor palsies with internal carotid-posterior communicating artery aneurysms. Ann Neurol. 1983;13:149-54. 22. Thompson HS, Pilley SFJ. Unequal pupils. A flow chart for sorting out the anisocorias. Survey of Ophthalmology. 1976;21(1):45-8. 23. Lemos J, Eggenberger E. Neuro-ophthalmological emergencies. The Neurohospitalist. 2015; 5(4):223-33. |

