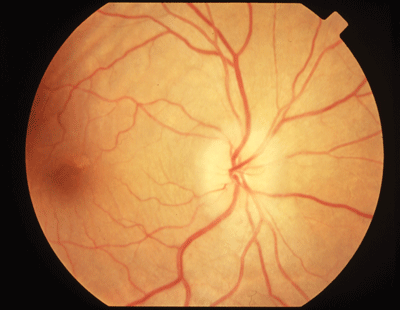
Best-corrected acuity in the left eye is 20/200. She has a relative afferent pupillary defect and greatly diminished color perception. A dilated examination reveals a normal appearance of the optic disc in that eye.
At this point, we determine that she has a retrobulbar optic neuropathy in the left eye. Subsequent magnetic resonance imaging (MRI) of the brain, orbits, and chiasm reveals multiple periventricular white-matter lesions. The diagnosis is now clear: optic neuritis secondary to multiple sclerosis (MS)
|
|
|
Papillitis is seen in this patient who has demyelinating optic neuropathy secondary to MS. |
Early in our training and careers, we wouldnt have approached this patient as we would today. Back then, once we suspected optic neuritis, we ordered serologic testing to rule out occult infectious disease and a computed axial tomography (CAT) scan to rule out orbital or intracranial mass. Thus, we diagnosed optic neuritis by excluding everything else.
We did not order the more definitive MRI for two reasons. First, MRI was not yet commonly used in eye care. Second, we did not want to actually diagnose MS. Once we felt confident that the diagnosis was optic neuritis, we typically stopped there.
Whenever we diagnosed MS, the patients faced an uncertain prognosis. If they were uninsured, this diagnosis would likely have prevented them from getting full medical coverage in the future. In fact, a diagnosis of optic neuritis was all that was necessary for an insurance carrier to refuse to cover a patient.
However, the most compelling reason that we did not want to diagnose MS comes from an old adage: There is no reason to rush to a fire if you have no hoses to put out the fire.
While there were no effective treatments for MS when we first started our careers, we now have options. In this months column, we explore the medical treatments available to put out the fires of multiple sclerosis.
MS Overview
MS is a multifactorial, demyelinating disease that affects white matter in the central nervous system (CNS). In MS, the Schwann cells that surround the axons become disrupted. When this myelin sheath is disrupted, saltatory conduction of the nerve impulse is disturbed, resulting in motor or cognitive impairment.
One theory concerning the mechanism of demyelinating disease implicates the participation of immunoregulatory cytokines.1 These immunoregulatory cytokines are thought to accumulate within the CNS, where they attack and destroy the myelin sheath, thus contributing to the demyelination process.
MS demonstrates a pattern of relapses and remissions, with the accumulation of disability over time.
MS Treatment
Beginning MS treatment as soon as possible is very important; over time, nerve damage caused by multiple sclerosis in the brain and spinal cord becomes permanent and leads to physical disability or cognitive impairment. The treatment of MS focuses mainly on decreasing the rate and severity of relapse, reducing the number of MS lesions, delaying the progression of the disease, and providing symptomatic relief for the patient. (A discussion of the symptoms, relapses and remissions of MS is beyond the scope of this column.)
 |
| Over time, nerve damage caused by MS in the brain and spinal cord becomes permanent and leads to physical disability or cognitive impairment. |
Advances in interferon therapy have assisted greatly in the management of impairment from MS. Interferons are substances produced by the body to combat viral infections and regulate the immune system.
Recombinant interferon therapies have proven to be effective in reducing relapses and accumulation of brain lesions over time.2-10 The overall reduction in risk of relapse appears to be nearly 30%.2 Patients who have a lower baseline relapse rate prior to the initiation of interferon therapy seem to have a more favorable prognosis.4 Adverse reactions associated with these medications include flu-like symptoms, increased spasticity of lower limbs, site injection reactions and systemic allergic reactions.7
All MS treatments are given by injection. There are currently four available MS drugs:
Avonex (interferon beta-1a, Biogen Idec). This is indicated for patients who have relapsing forms of MS to slow the accumulation of physical disability and decrease the frequency of clinical exacerbations. Avonex appears to be most successful in patients who have experienced a first clinical episode of focal neurologic deficit and have MRI features consistent with MS.4
Avonex is injected intramuscularly once a week. It decreases the rate of relapse and the development of new lesions. It also delays the progression of motor and cognitive disability.
Rebif (interferon beta-1a, Serono and Pfizer). Similar to Avonex, this interferon is injected subcutaneously three times per week. Rebif also reduces the rate of relapse and slows the progression of disability.3
Betaseron (interferon beta-1b, Berlex). This drug is injected subcutaneously three to four times per week. It has similar indications and actions of Avonex and Rebif.4,5
Copaxone (glatiramer acetate, Teva Pharmaceutical). This is actually a mixture of amino acids. It is injected subcutaneously seven times per week. Exactly how Copaxone works is unclear, but it is believed to modify the immune process that causes MS.7 In one study, nearly all patients who used Copaxone (with a mean disease duration of 15 years) remained ambulatory after 10 years.10 Patients who withdrew from treatment had greater disability than patients who continued treatment.10
Patients can develop resistance to these drugs if they endogenously produce neutralizing antibodies that inactivate MS therapy. Although the significance of neutralizing antibodies is not fully understood, it is thought that they can render MS treatments less effective over time.11 So, the potential for development of neutralizing antibodies is an important consideration in determining whether to initiate treatment.
Optic neuritis has long been seen as a calling card of MS. While we typically suspected underlying MS in patients who had optic neuritis, we were averse to actually diagnosing MS in these patients years ago because we could offer them no effective treatment. Today, however, we have several therapies that reduce the relapse rate of the disease and slow the progression and development of both motor and cognitive disabilities. Because the clinical course of MS can now be altered, it is imperative to diagnose the patient as quickly as possible so that immunomodulatory therapies can be offered.
1. Wang HY, Matsui M, Araya S, et al. Chemokine receptors associated with immunity within and outside the central nervous system in early relapsing-remitting multiple sclerosis. J Neuroimmunol 2002; Dec;133(1-2):184-92.
2. Le Page E, Edan G. Disease modifying therapy drugs approved in multiple sclerosis. Rev Prat 2006 Jun 30;56(12):1336-46.
3. Portaccio E, Zipoli V, Siracusa G, et al. Response to interferon-beta therapy in relapsing-remitting multiple sclerosis: a comparison of different clinical criteria. Mult Scler 2006 Jun;12(3):281-6.
4. Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing-remitting multiple sclerosis. Acta Neurol Scand 2006 May;113(5): 283-7.
5. Khan OA, Tselis AC, Kamholz JA, et al. A prospective, open-label treatment trial to compare the effect of IFN beta-1a (Avonex), IFNbeta-1b (Betaseron), and glatiramer acetate (Copaxone) on the relapse rate in relapsing-remitting multiple sclerosis. Eur J Neurol 2001 Mar;8(2):141-8.
6. Haas J, Firzlaff M. Twenty-four-month comparison of immunomodulatory treatmentsa retrospective open label study in 308 RRMS patients treated with beta interferons or glatiramer acetate (Copaxone). Eur J Neurol 2005 Jun;12(6):425-31.
7. Flechter S, Vardi J, Pollak L, et al. Comparison of glatiramer acetate (Copaxone) and interferon beta-1b (Betaferon) in multiple sclerosis patients: an open-label 2-year follow-up. J Neurol Sci 2002 May 15;197(1-2):51-5.
8. Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006 Aug 30; [Epub ahead of print].
9. Patti F, Pappalardo A, Florio C, et al. Effects of interferon beta-1a and -1b over time: 6-year results of an observational head-to-head study. Acta Neurol Scand 2006 Apr;113(4):241-7.
10. Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler 2006 Jun;12(3): 309-20.
11. Kappos L, Clanet M, Sandberg-Wollheim M, et al. Neutralizing antibodies and efficacy of interferon beta-1a: a 4-year controlled study. Neurology 2005 Jul 12;65(1):40-7.