 |
In all medical specialties, doctors have long relied on glucocorticoid therapy. However, it’s well-known that systemic glucocorticoid treatments (e.g., oral prednisone) affect other cells in the body. In some patients, these drugs can produce side effects that may include diabetes, impaired wound healing, gastric ulcers, weight gain, cardiovascular disease, bone loss, muscle wasting and neuropsychiatric issues.1-3 We also know that glucocorticoids create additional adverse effects in the eye, including increased intraocular pressure and potential glaucoma, cataract formation, central serous chorioretinopathy, corneal thinning, increased risk of infection and impaired wound healing.1
Unlike steroids, the innate melanocortin (MC) pathways help the immune system regulate itself within the ocular microenvironment. For example, aqueous humor suppresses activation of immune cells that mediate hypersensitivity.1,4 In addition, aqueous humor-treated immune cells suppress hypersensitivity-mediating T-cells.1,5 Because the α-melanocyte-stimulating hormone (α-MSH) is present in the aqueous humor, it helps prevent the potential damages of inflammation.
Beyond the aqueous humor, cells in the iris, ciliary body and retinal pigment epithelium also play a role in immune privilege and inflammation suppression relative to the MC pathways.1 Given these distinct advantages and the fact that all five MC receptors affect the eyes, α-MSH-based treatments are a reasonable alternative to glucocorticoid therapy worthy of our consideration.
 |
|
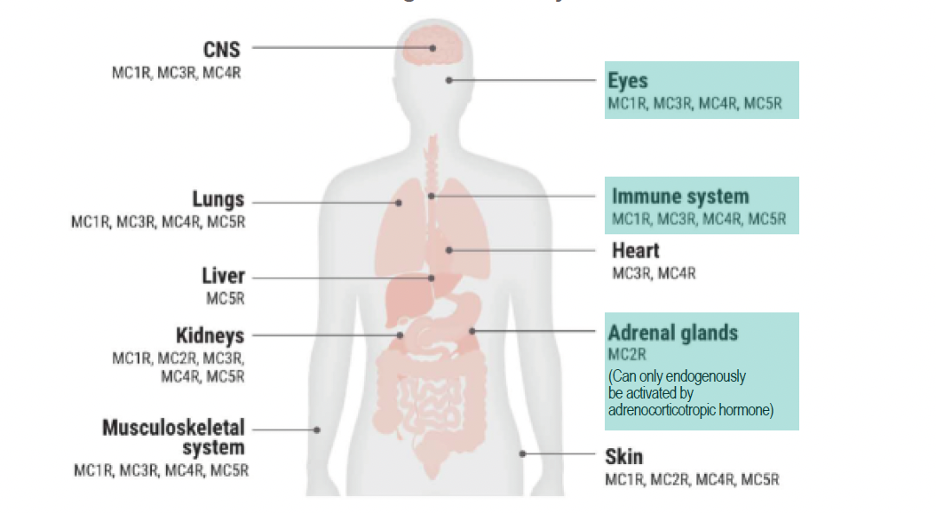
Fig. 1. The various MC receptors throughout the body. Photo: Taylor AW, et al. Click image to enlarge. |
The Melanocortin System
This neuronal pathway is made up of a collection of multiple peptides, including the adrenocorticotropic hormone (ACTH), as well as five MC receptors, which are expressed in various cells and tissues throughout the body (Figure 1).1 The MC system controls inflammation by releasing corticotropin-releasing hormone, which in turn stimulates pro-opiomelanocortin production, thereby generating the MC peptides.1 MCs have many functions, which are carried out when they bind to one of five known MC receptors.1 Each of these receptors is expressed in a specific cell type.1 The adrenocorticotropic hormone, in particular, stimulates the MC receptor 2 on the adrenal cortex, which thereby produces glucocorticoids. The other four MC receptors are found in the eye (Figure 2). Glucocorticoids have anti-inflammatory effects, but they also act as immune modulators.1
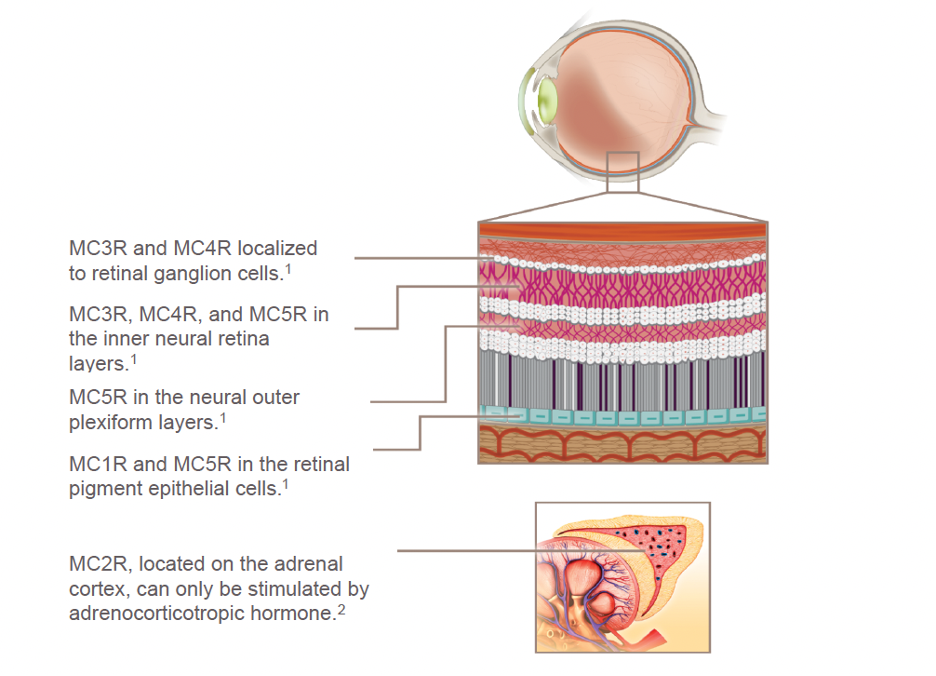 |
| Fig. 2. MC receptors are expressed by immune cells, as well as in ocular cells, retinal pigment epithelial cells, retinal ganglion cells and the neural outer plexiform layer. Photo: Catania A, et al. Click image to enlarge. |
MC Pathway-based Treatment
Repository corticotropin injection (RCI) is a highly purified formulation of ACTH in 16% gelatin that provides sustained release after subcutaneous (or intramuscular) injection.6 The most common RCI—Acthar Gel (Mallinckrodt Pharmaceuticals)—was first FDA approved in 1952; yet, for decades, it hasn’t been commonly used due to potential reasons such as poor marketing, assumptions that the product is outdated and the requirement of self-administered biweekly injections. Given new, promising research on RCI and considering the known potential adverse effects of traditional steroids, this form of therapy is becoming more frequently used in multiple areas of medicine, including optometry.
Acthar engages MC receptors expressed in immune, organ and tissue cells throughout the body and is thought to produce both an indirect anti-inflammatory effect and a direct cell modulation effect (Figure 3).6-11 The drug is approved for 19 indications across nine disease categories.12 For example, in neurology it’s used for the treatment of infantile spasms in infants and children under two years of age as well as for acute exacerbations of multiple sclerosis in adults. In pulmonology, it’s used to treat symptomatic sarcoidosis. In nephrology, it’s used to induce a diuresis or a remission of proteinuria in nephrotic syndrome. In rheumatology, it can help patients who have arthritis or lupus erythematosus.
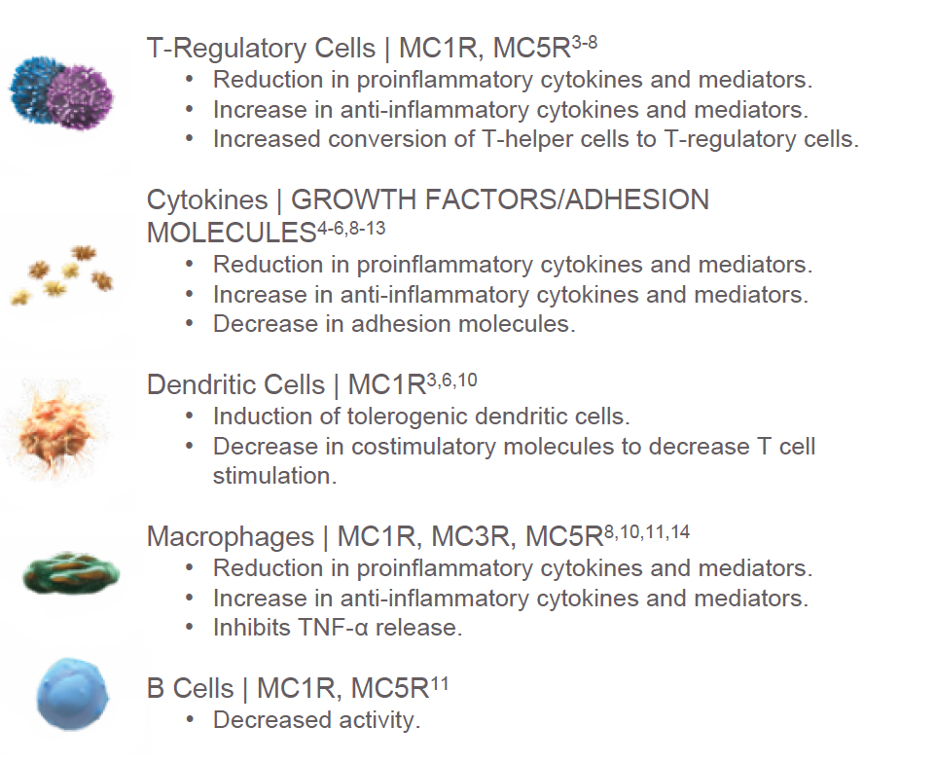 |
| Fig. 3. Engaging all five MC receptors may have an impact on immune cells and cytokines. Photo: Ahmed TJ, et al. Click image to enlarge. |
Importantly, we can also turn to this therapy to treat patients with ocular disease. Currently, Acthar Gel is indicated for the treatment of severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa, such as keratitis, iritis, iridocyclitis, diffuse posterior uveitis and choroiditis, optic neuritis, chorioretinitis and anterior segment inflammation.6,13-17
Eyecare practitioners don’t need to be responsible for administering the injections or teaching patients how to inject themselves. Mallinckrodt offers a service where nurses can provide the initial injections at a patient’s home and show them how to do it on their own.
Contraindications include patients with uncontrolled hypertension or uncontrolled diabetes. Patients should be educated regarding the low incidence but potential for insomnia or, alternatively, lethargy. Since patients are typically administering biweekly subcutaneous injections themselves, there is always the potential of injection site irritation. Research shows that Acthar produces about 3% of the systemic cortisol effect compared with systemic prednisone.18 In my opinion, since it’s a combination of ACTH and other natural pituitary peptides, it essentially “resets” the immune system in helping patients with treatment-resistant inflammatory eye conditions, including keratitis, uveitis and keratoconjunctivitis sicca (KCS).
Takeaways for Optometry
In a recent study that observed 91 patients with a several-year history of uveitis that did not adequately respond to previously attempted treatments, most patients showed improvement after initiating Acthar.6 In another study, 35 patients with severe, treatment-resistant KCS received Acthar twice weekly for 12 weeks and showed significantly reduced corneal and conjunctival staining, improved dry eye symptoms and, most impressively, improved quality-of-life scores at two, four, six and 12 weeks.19 Few drugs have shown this level of efficacy on quality of life using the 57-item IDEEL questionnaire (Alcon), especially with such minimal side effects.
Given Acthar’s safety profile and the challenges we often face in treating many of the conditions for which it is indicated, we should consider it in three circumstances, which I refer to as the “three Rs”—resisters, rebounders and responders. The first group of patients who may be candidates for Acthar Gel include those who are resistant to treatment, meaning you’ve tried multiple treatment options that failed to resolve the keratitis or uveitis. The second group consists of patients who experience rebound keratitis or uveitis after you taper or discontinue their medications, such as topical steroids. The third group of patients who may benefit from treatment with Acthar Gel are steroid responders. In one study, 50% of patients with uveitis were off steroids by six weeks, and 80% no longer required steroids after three months following Acthar initiation.6,20
Consider Acthar a later option in your armamentarium for recalcitrant cases of keratitis, including KCS, for uveitis management in steroid responders and for keratitis or uveitis rebounders. Many patients who also suffer from autoimmune conditions seem to do well ocularly and systemically with Acthar. It can be a valuable tool for those patients with few remaining options.
Dr. Karpecki is the director of Cornea and External Disease for Kentucky Eye Institute, associate professor at KYCO and medical director for Dry Eye Institutes of Kentucky and Indiana. He is the Chief Clinical Editor for Review of Optometry and chairman of the affiliated New Technologies & Treatments conferences. A fixture in optometric clinical education, he provides consulting services to a wide array of ophthalmic clients. Dr. Karpecki’s full disclosure list can be found here.
1. Clemson CM, Yost J, Taylor AW. The role of Alpha-MSH as a Modulator of ocular immunobiology exemplifies mechanistic differences between melanocortins and steroids. Ocul Immunol Inflamm. 2017;25(2):179-89. 2. Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002:96(1):23-43. 3. Harris E, Tiganescu A, Tubeuf S, et al. The prediction and monitoring of toxicity associated with long-term systemic glucocorticoid therapy. Curr Rheumatol Rep. 2015:17(6):513. 4. Kaiser CJ, Ksander BR, Streilein JW. Inhibition of lymphocyte proliferation by aqueous humor. Regional Immunology. 1989:2(1):42-9. 5. Nishida T, Taylor A. Specific aqueous humor factors induce activation of regulatory T cells. Investigative Ophthalmology & Visual Science. 1999:40(10):2268-74. 6. Nelson WW, Lima AF, Kranyak J, et al. Retrospective medical record review to describe use of repository corticotropin injection among patients with uveitis in the United States. J Ocul Pharmacol Ther. 2019;35(3):182-88. 7. Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. ScientificWorldJournal. 2010;10:1840-53. 8. Wright D, Fitch R. Repository corticotropin injection (H.P. Acthar Gel) enhances remyelination after cuprizone-induced demyelination. NDM04. Int J MS Care. 2019a;21(suppl 1):61-62. 9. Olsen NJ, Decker DA, Higgins P, et al. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300. 10. Healy LM, Jang JH, Lin YH, Rao V, Antel JP, Wright D. Melanocortin receptor-mediated anti-inflammatory effect of repository corticotropin injection on human monocyte-derived macrophages. Mult Scler J. 2017;23(suppl 3):777. 11. Wright D, Zweifel B, Sharma P, Galen K, Fitch R. Reduced steroidogenic activity of repository corticotropin injection induces a distinct cytokine response following T cell activation in vivo (EULAR abstract AB0082). Ann Rheum Dis. 2019b;78(suppl 2):1504. 12. Acthar Gel. Repository corticotropin injection (Prescribing Information). Mallinckrodt ARD LLC. 13. H.P. Acthar Gel (repository corticotropin injection) prescribing information. Mallinckrodt Pharmaceuticals. 2017. 14. ClinicalTrials.gov. Adrenocorticotropic hormone (ACTH) for post-op inflammation in proliferative vitreoretinopathy (PVR). Updated August 10, 2022. https://clinicaltrials.gov/ct2/show/NCT03727776. Accessed July 20, 2022. 15. ClinicalTrials.gov. Clinical efficacy of H.P. Acthar Gel 80 U/mL to improve the signs and symptoms in subjects with dry eye disease. January 28, 2021. https ://clinicaltrials.gov/ct2/show/NCT03287635. Accessed July 20, 2022. 16. ClinicalTrials.gov. A clinical study to evaluate the potential role of ACTH Gel in patients with scleritis (ATLAS). Updated July 12, 2022. https://clinicaltrials.gov/ct2/show/NCT03465111. Accessed July 20, 2022. 17. ClinicalTrials.gov. Efficacy and safety of H.P. Acthar Gel in adults with retinal vasculitis. September 12, 2019. https://clinicaltrials.gov/ct2/show/NCT03066869. Accessed July 20, 2022. 18. Bomback AS, Tumlin JA, Baranski J, et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther. 2011;5:147-53. 19. Grieco J, McLaurin EB, Ousler GW, et al. Topline results from a multicenter, open-label, phase 4 study of repository corticotropin injection in patients with treatment-resistant severe non-infectious keratitis. Invest Ophthalmol Vis Sci. 2021;62(8):1275. 20. Madan A, Mijovic-Das S, Stankovic A, et al. Acthar Gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016;17:37. |

