History
A 54-year-old white female presented with a chief complaint of gradual vision loss in her left eye that had persisted since her most recent examination two years earlier. At that exam, her eye care provider suggested that she had “bleeding in her eye” and needed to see a retinal specialist.
 |
Her systemic history was significant for hypertension, myocardial infarction, hypercholesterolemia, gastroesophageal reflux disorder, arthritis and anemia. She explained that, after a lengthy delay, she made an appointment with us following a recommendation from her general physician.
Current medications included 5mg amlodipine besylate, 3.125mg carvedilol, 75mg clopidogrel, 100mg losartan, 40mg pantoprazole, 20mg simvastatin and low-dose (81mg) aspirin. She reported no known allergies of any kind.
Diagnostic Data
Her best-corrected visual acuity measured 20/20 OD and 20/300 OS, with no improvement upon pinhole testing. Pupils were equal, round and reactive to light, with no evidence of afferent defect OU.
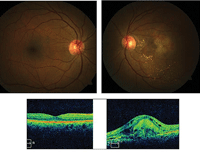 |
|
|
Fundus and optical coherence tomography images of our 54-year-old patient who reported vision loss in her left eye (OD top/bottom left, OS top/bottom right).
|
Extraocular muscle movements were full and smooth. Confrontation fields were full to finger counting in each eye, with a blurry and distorted facial Amsler result in the left eye.
Slit lamp examination was remarkable for bilateral corneal arcus. Intraocular pressure measured 14mm Hg OU, and her blood pressure was 107mm Hg/89mm Hg.
Your Diagnosis
How would you approach this case? Does the patient require any additional tests? What is your diagnosis? How would you manage this patient? What is the likely prognosis?
Discussion
Additional testing included a standard Amsler grid test to quantify the extent of the visual distortion in her left eye. Additionally, we ordered an optical coherence tomography (OCT) scan and referred her to a retinal specialist for a fluorescein angiography (FA).
The diagnosis in this case is exudative age-related macular degeneration (AMD).
AMD is a complex, progressive, degenerative disease involving multiple genetic, systemic, lifestyle and environmental factors.1-15 It is the leading cause of acquired blindness in elderly individuals in Europe and North America.1-5 As the disease progresses through the non-exudative (dry) form, metabolic triggers cause the release of vascular endothelial growth factor (VEGF).1,13-19 This produces the choroidal neovascularization that defines exudative (wet) AMD.6-10 Approximately 30% of adults aged 75 years or older have some signs of dry AMD, with 6% to 8% of these individuals experiencing significant vision loss during more advanced disease stages.7
The hallmark features of non-exudative AMD––drusen, retinal pigment epithelial (RPE) changes, choroidal thinning and vitreoretinal adhesion––prime the tissues for hypoxic stress, causing hypoxia and vascular endothelial growth factor accumulation.19-24 These mechanisms cause the RPE to degenerate, resulting in photoreceptor loss. As the photoreceptors disintegrate, the inner nuclear layer collapses causing it to contact Bruch’s membrane, initializing degeneration of the outer retinal layers.2-5 Ultraviolet radiation-induced oxidation and free radical formation within these structures occurs concurrently.25 Genetic predisposition, nutrient-deficient dietary intake, obesity, smoking and many cardiovascular factors are all associated with the pathogenesis of dry AMD.1
Wet AMD results when the RPE/Bruch’s barrier becomes compromised by new, weak and leaky blood vessels that grow in the choreocapillaris. When hypoxic levels cross the barrier, fluid effusion and neovascularization result.19,22-24 These occult or classic sub-retinal choroidal neovascular membranes (CNV) leak serosanguinous fluid, precipitating RPE detachment, sensory retinal detachment, subretinal or intraretinal bleeding, fibrovascular, disciform scarring and geographic choroidal atrophy.1-25
The best management for dry AMD generally involves early detection and monitoring, as well as lifestyle and dietary alteration. This process generally begins with a biannual dilated fundus examination and patient education about the benefits of smoking cessation and/or dietary supplementaiton.26,27 Researchers have shown that oral antioxidants, such as vitamins C and E and zinc, may help reduce early drusen formation by terminating the chemical reactions initiated by free radical production.26,27 Advanced vitamin formulations containing carotenoids, such as lutein, zeaxanthin and meso-zeaxanthin, and polyunsaturated fish oils also have been shown to slow progression from dry to wet AMD.26-28
The management of exudative AMD depends on the type, location and size of the neovascular lesions.29,30 Modern treatment options include the vascular endothelial growth factor inhibitors Avastin (bevicizumab, Genentech/Roche), Lucentis (ranibizumab, Genentech/Roche) Eylea (aflibercept, Regeneron) and Macugen (pegaptanib, Valeant), which may be administered as monotherapy or in combination with laser and photodynamic therapy.29 By interfering with VEGF’s effects (i.e., increased vascular permeability, angiogenesis and induced microvessel formation), disease progression can be limited or even reversed.29,31
Multiple studies have shown that intravitreal anti-VEGF injections are superior to verteporfin photodynamic therapy in the treatment of predominantly classic CNV secondary to wet AMD.31 Other studies, including ANCHOR and MARINA, have shown that anti-VEGF therapy also is effective for the treatment of occult CNV lesions associated with neovascular AMD.31
Monthly anti-VEGF injections generally are well tolerated, and demonstrate low rates of ocular and systemic adverse events.31 Less frequent dosing has been evaluated in a strategy known as “treat and extend,” with the goal of reducing the inconvenience, risk and cost of monthly treatment.31
In cases where bilateral central visual acuity has been compromised, low vision and vision rehabilitation specialists may be able to offer training, optical devices and other equipment that will improve patients’ quality of life and activities of daily living. It is important to note that wet AMD patients who have already gone blind in one eye have a 10% risk of developing wet AMD in the fellow eye if no large drusen or pigment clumps are present; a 30% risk if either large drusen or pigment clumps are present; and a 50% risk if both pigment clumps and large drusen are present.1,6
Currently, several companies offer genetic testing for AMD, including Arctic Dx (Macula Risk PGx) and Nicox (RetnaGene). These tests are recommended for the immediate family members of high-risk patients, and may indicate the likelihood of developing AMD later in life and/or the potential protective benefits of early lifestyle intervention.
We referred the patient to a retinal specialist, who initiated intravitreal Avastin therapy in her left eye. Considering her two-year delay in seeking appropriate care, we also stressed the importance of routine follow-up visits to prevent future vision loss in her contralateral eye. At the time of publication, the patient had not yet returned to our office.
1. Klettner A, Kauppinen A, Blasiak J, et al. Cellular and molecular mechanisms of age-related macular degeneration: from impaired autophagy to neovascularization. Int J Biochem Cell Biol. 2013;45(7):1457-67.
2. Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013;54(14):68-80.
3. van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014;232(2):151-64.
4. Machalińska A. Age-related macular degeneration as a local manifestation of atherosclerosis––a novel insight into pathogenesis. Klin Oczna. 2013;115(1):74-8.
5. Turlea C. New aspects in age related macular degeneration. Oftalmologia. 2012;56(1):36-44.
6. Akpek EK, Smith RA. Overview of age-related ocular conditions. Am J Manag Care. 2013;19(5 Suppl):S67-75.
7. Tranos P, Vacalis A, Asteriadis S, et al. Resistance to antivascular endothelial growth factor treatment in age-related macular degeneration. Drug Des Devel Ther. 2013;7(1):485-90.
8. Maguire MG, Daniel E, Shah AR, et al. Incidence of choroidal neovascularization in the fellow eye in the comparison of age-related macular degeneration treatments trials. Ophthalmol. 2013;120(10):2035-41.
9. Novack GD. Pharmacotherapy for the treatment of choroidal neovascularization due to age-related macular degeneration. Annu Rev Pharmacol Toxicol. 2008;48 (1):61-78.
10. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab (Avastin) in combination with verteporfin photodynamic therapy for choroidal neovascularization associated with age-related macular degeneration (IBeVe Study). Graefes Arch Clin Exp Ophthalmol. 2007;245(9):1273-80.
11. Nano ME, Lansingh VC, Pighin MS, et al. Risk factors of age-related macular degeneration in Argentina. Arq Bras Oftalmol. 2013;76(2):80-4.
12. Mettu PS, Wielgus AR, Ong SS, Cousins SW. Retinal pigment epithelium response to oxidant injury in the pathogenesis of early age-related macular degeneration. Mol Aspects Med. 2012;33(4):376-98.
13. Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012;33(4):295-317.
14. Kinnunen K, Petrovski G, Moe MC. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90(4):299-309.
15. Roth F, Bindewald A, Holz FG. Key pathophysiologic pathways in age-related macular disease. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):710-6.
16. Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol. 2004;137(3):504-10.

