 |
History
A 68-year-old white male presented for an initial comprehensive ocular examination with a chief complaint of intermittent burning of both eyes over the past two years.
The patient’s secondary complaint was floaters in both eyes occurring daily for the last few years but stable. The patient denied any additional ocular history and reported a medical history of hypertension, gout and hypothyroidism, for which he was poorly medicated using lisinopril/hydrochlorothiazide, allopurinol, sertraline and ranitidine.
The patient was currently taking Synthroid (levothyroxine, AbbVie) and denied having any allergies to medications or environment.
Diagnostic Data
His best-corrected entering visual acuity was 20/20 OU. His external examination was unremarkable with no evidence of afferent pupillary defect. Biomicroscopic evaluation of the anterior segment showed mild exposure keratopathy adjacent to bilateral pterygia. Goldmann applanation tonometry measured 17mm Hg OU. The pertinent retinal findings are demonstrated in the photographs.
Your Diagnosis
Does the case presented require any additional tests, history or information? What steps would you take to manage this patient? Based on the information provided, what would be your diagnosis? What is the most likely prognosis?
Discussion
The diagnosis in this issue is inactive chorioretinal scarring secondary to an old retinal event involving a presumed organism of Toxocara species.
Thousands of parasites live among humans. Countries across the world differ vastly in environment, sanitation and access to healthcare, and parasites are commonly encountered.
Globally, parasitic roundworms such as Toxocara canis and Toxocara cati cause substantial systemic damage to a multitude of organs, including the heart, lungs, brain, liver and eyes, among others. 1-5 The eggs of these roundworms (commonly found in gastrointestinal helminth of cats and dogs) are transmitted to humans via direct contact resulting in the infection known as Toxocariasis.1-2,4-5
Toxocariasis is the most common human parasitic worm infection within the United States and worldwide. It is considered to be an underdiagnosed condition; it is estimated that millions of persons worldwide have been exposed to the Toxocara parasite.6-10 The national seroprevalence of Toxocara species infection has been estimated to be 13.9% in the U.S. population over the age of six.11 Specifically, the prevalence has been found to be higher in non-Hispanic Blacks (21.2%) than non-Hispanic Whites (12%) and Mexican-Americans (10.7%), with a higher association amongst those with less than a high school education.11
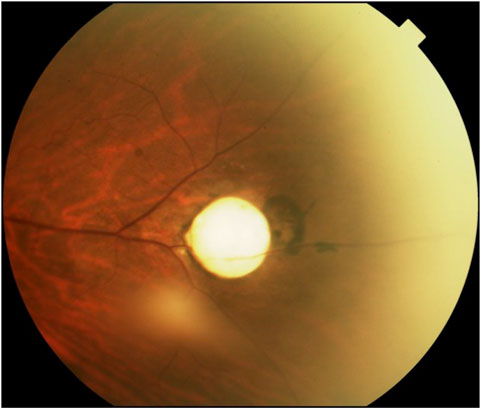 |
| This fundus photograph shows a Toxocara-induced granuloma with accompanying retinal pigmentation. |
On the global scale, human toxocariasis has been found to be more prevalent in tropical settings than in temperate regions, due to the inability of Toxocara larvae to develop at temperatures below 50°F (10°C).3,12,13 Egg embryonation occurs year-round in tropical climates, whereas there is only seasonal egg embryonation in temperate zones, which explains the prevalence distribution in more detail.14 Although globally toxocariasis is more prevalent in rural communities than urban communities, persons in both poor urban and poor rural areas in the United States, with a concentration in the South and Appalachian regions demonstrate higher rates of infection secondary to extensive numbers of Toxocara-infected dogs and cats reported in these regions.10-13,15-17 Rates of exposure are persistently higher in children and associated with dog ownership, poor hygiene and lack of sanitary facilities.4,6
Humans become infected after unintentionally ingesting embryonated Toxocara eggs. These are most commonly found in soil, plants, or soil-dwelling invertebrates contaminated with fecal matter shed by infected animals.4,12,18 Although rare, humans may additionally become infected after consumption of improperly cooked meat, organs, or unwashed vegetables containing Toxocara eggs or larvae.1,10 After ingestion, the eggs release larvae into the small intestine where they penetrate the intestinal wall, enter the circulation, and subsequently migrate to various organs inducing autoimmune inflammatory reactions and a wide variety of patient symptoms.1,6,14 The liver, lungs and eyes are the most commonly affected organs with occasional central nervous system involvement being reported.2,6,12,19
Toxocariasis, depending on the number of parasites released into the circulation, the duration of larva migration, intensity of infection and host immune response, can result in a variety of syndromes with multiple clinical manifestations.2,6,14,20 Originally toxocariasis was separated into two classifications: visceral larval migrans (VLM) syndrome and ocular larval migrans (OLM) syndrome.4 These terms are nonspecific and can be misleading, as clinical manifestations of other diseases can be caused by migrating larva.4 The terms visceral toxocariasis (VT) and ocular toxocariasis (OT) are more specific to the condition.4 With improved understanding of the disease as a result of enhanced diagnostic testing and seroepidemiological screening surveys, additional syndromes have been recognized and include: covert toxocariasis, common toxocariasis and neurotoxocariasis.6,14
The vast majority of persons with Toxocara species infections go undiagnosed as they are asymptomatic or maintain mild, nonspecific symptoms.4,14 Labeled both “covert” and “common” toxocariasis these patients can display a wide variety of vague symptoms such as cough, abdominal pain, sleep disturbances, behavioral changes or headaches, or both. 4,6,14,21
Visceral toxocariasis is primarily diagnosed in young children under the age of eight and is associated with larval infection that can persist for many months.4,14 Although there is limited knowledge of the incidence of VT, the condition results when parasites infect various organs or systems, most frequently the liver, lungs and central nervous system.2,4 Accordingly, classical signs and symptoms include fever, respiratory problems (coughing/wheezing), muscle pain, fatigue, neurological manifestations and hepatosplenomegaly.2,4,6,14 Patients with VT typically have elevated Immunoglobulins E(IgE), G(IgG), M(IgM) and leukocytosis, specifically eosinophilia with concentrations of white blood cells in excess of 2000 cells/mm3.2,6
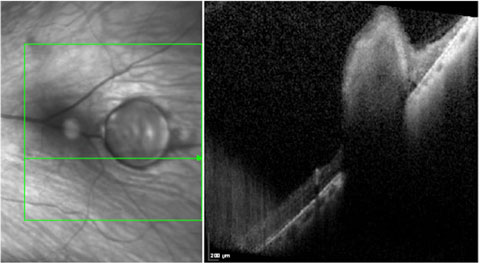 |
| This SD-OCT cross section shows a granuloma secondary to Toxocara spp. Note the superficial and internal hyperreflectivity of the lesion with induced shadowing of the underlying structures, all consistent with a granuloma. |
Ocular toxocariasis (OT) is considered relatively uncommon in comparison to VT, with limited statistics on the prevalence. The majority of cases of this form of the disease occur in the United States.1,3,14 The results of a survey of ophthalmologists and optometrists in Alabama demonstrated rates of OT of 1 and 11 cases per 1,000 persons respectively.22
Worldwide, OT affects both adults and children with reported mean age of onset ranging from 6.4 to 51.7 years.1 In the United States OT is most frequently diagnosed in children, with studies reporting OT in persons between 2 and 16 years of age.1-4,23 Adult OT has been found to be more prevalent in Japan and South Korea with a mean age of onset >20 years of age.23 This has been attributed to the consumption of raw foods.23 Patients with OT are generally healthy with normal blood counts, without elevated IgE levels as seen in the VT form.21,24
Most often, OT is unilateral (bilateral cases are extremely rare).2,4,25 OT occurs when Toxocara larvae migrate through the circulation into the posterior segment of the eye triggering a subsequent ocular immune response.3 A wide spectrum of ophthalmological symptoms may exist, the most common of which is vision loss (85%).3,18 Pediatric patients often present with eye redness, eye pain, photophobia, leukocoria and strabismus.4
Clinical examination findings are classified into four groups: posterior pole granuloma, peripheral granuloma, nematode endophthalmitis and atypical presentations.1-3,25
Posterior pole granuloma is the most common manifestation, presenting in 25%-50% of cases.26 It consists of a white, focal, sub or intra-retinal inflammatory mass in the posterior pole usually less than or equal to one disc diameter in size with variable accompanying pigmentation, active inflammation and vitreal involvement.1,3 Accompanying vision loss can result from direct involvement of the optic disk or macula, or by development of epiretinal membrane, retinal folds, or choroidal neovascular membrane (CNV).26,27
Peripheral granulomas have been historically reported in 20%-40% of patients.28 These are focal, white, elevated masses in the peripheral retina (90% temporally) which can present with vitreal traction membranes and retinal folds, pigmentary changes and retinal detachment.1,2,28
Nematode endophthalmitis produces a panuveitis manifesting as a red, painful eye with long term sequelae ranging from cyclitic membranes to retinal detachment.1-3 Endophthalmitis occurs in up to 25% of cases and is found more frequently in patients under the age of eight.1,2,28
Atypical presentations are those which do not fall into the other three categories. These cases can present with signs and symptoms that include optic neuritis, uveitis (comprises 1% to 2% of uveitis in under seven age group), cataracts, diffuse unilateral subacute neuroretinitis, motile subretinal larvae and diffuse chorioretinitis.1,2,29-32
Diagnosis
The definitive diagnosis of toxocariasis is made through detection of Toxocara larvae during histological evaluation of infected tissues.6,14,19 This is, however, invasive and rarely justifiable nor routinely attempted, as the diagnosis of toxocariasis is most often made clinically.1,2,6,19 Clinically the diagnosis is made based on clinical presentation, medical history and positive serological antibody testing.25 The recommended test for presumptive toxocariasis is an indirect enzyme-linked immunosorbent assay (ELISA) which identifies excess levels of Immunoglobulin G (IgG) produced in response to the excretory-secretory antigens of Toxocara larvae.2,7 An ELISA titer greater than 1:32 has a diagnostic sensitivity of 78% and specificity of greater than 90% for toxocariasis.2,7 Notably, ELISA titers can remain elevated for years, confounding whether there is active infection or past exposure.7 Although not as helpful as IgG, systemic eosinophilia is an important feature associated with Toxocara infection.1,5 Hence serum immunoglobulin E (IgE) or eosinophil count should be obtained as these levels may be useful in confirming systemic toxocariasis; additionally the values can indicate treatment success as the numbers depress.1,5
Standard diagnosis of OT is made based on a clinical presentation consistent with the disease and positive serum antibodies to Toxocara larvae.3 OT is considered more difficult to diagnose with serological testing than VT due to low or absent antibody titers.1,7 It has been suggested that a serum titer of 1:8, coupled with signs and symptoms consistent with OT, is acceptable to support a diagnosis of OT.25 In a2014 study, data revealed that serum IgE level was elevated in approximately 70% of OT patients, reinforcing the utility of that bloodwork.5
In atypical situations, screening of aqueous humor and vitreous for antibodies using western blot has been shown to be more specific than using ELISA screening of serum antibodies in isolation for diagnosis.33
Ocular imaging can be powerful in the diagnostic evaluation of OT. Fluorescein angiography, ophthalmic ultrasound, ultrasound biomicroscopy and optical coherence tomography (OCT) are all useful.2,31 OCT is the least invasive technique and has been shown to be valuable in diagnosing and sequentially tracking epiretinal membrane formation, macular thickening, focal macular retinal detachments and additional tractional forces on the retina.2,34 Recently it has been shown that high-penetration OCT (HP-OCT) is more useful than conventional SD-OCT in examining chorio-retinal lesions secondary to Toxocara.35 HP-OCT employs a longer wavelength that provides enhanced resolution and penetration, providing clearer and more continuous images from the retina to the sclera-choroidal interface.35
Treatment
Ocular toxocariasis can lead to severe functional limitations as a result of damage to ocular structures from severe vitritis, cystoid macular edema, granuloma and retinal detachment.2,20,21,28 Long term outcomes can depend on the treatment selected and the timeliness of that treatment.33
Treatment for toxocariasis differs depending on the clinical type and severity of symptoms. Systemic toxocariasis is typically treated with corticosteroids and antihelmintic therapy.2 The recommended antihelmintic drug used for systemic toxocariasis is albendazole 400 mg BID x 7-14 days.1 Other medications with similar efficacies include mebendazole, thiabendazole and diethylcarbamazine.2,21
No consensus exists on the appropriate treatment regimen for OT as most recommendations are anecdotal or come from case reports or series; there are no reported clinical trials addressing the subject.2 Corticosteroid therapy, both topically and systemically, has been employed to reduce inflammation or prevent a severe inflammatory reaction secondary to dying larvae.1-2,21 Concurrent use of steroids and antihelminitic therapy is controversial with very few published studies supporting this treatment regimen.21,24,36 In a small, yet formative study published in 2001, combined treatment of albendazole and systemic prednisolone was given to five patients (seven eyes) to treat uveitis secondary to toxocariasis.21 All patients had resolved inflammation and improved visual acuity which suggested that combined steroid and antihelmintic use is effective in treating ocular toxocariasis.21
Surgical intervention may be necessary for patients with structural complications resulting from OT.1 Epiretinal membrane formation with vitreo-macular or papillary traction, persistent vitreous opacities and/or hemorrhage, retinal detachment are all indicators for vitreoretinal surgery.2,20 A 2011 study shows that surgical treatment (pars plana vitrectomy, pars plana lensectomy or combination of the two) of 45 patients with severe complications from OT resulted in satisfactory anatomic results and might improve visual outcomes.20 Laser photocoagulation has been shown to be effectively employed in a case where a motile subretinal larva was visualized.37 Intravitreal Ranibizumab, widely employed for the treatment of choroidal neovascularization (CNV) has successfully been used to treat a rare case of CNV in a 13-year-old boy with OT. 38 Lastly, cryotherapy can be delivered as an alternative treatment for peripheral granulomas.2
Prevention
Recognition of toxocariasis as the most common human parasitic worm infection in the U.S. and understanding that it is preventable is critical to long-term prevention of the disease.4,9 In addition to public awareness of the problem, education on how to directly prevent the condition is paramount. It is important to emphasize that less than 50% of person with ocular toxocariasis have reported owning a pet, in spite of dogs and cats being the principle carriers and transmitters of the disease.1 There are an estimated 90 million cats and 73 million dogs in the United States alone, some of whom are born with congenital toxocariasis.9 Minimally pet owners should be educated on the necessity of routine veterinary care for deworming (especially in young animals whom have a higher likelihood of disease), proper disposal of feces and maintenance of their living area so as to reduce the risk of spreading this disease.9 Given this disease’s preponderance to effect children, parents should be educated on reinforcing proper hygiene habits with their children, especially when playing in high-risk exposure areas frequented by both pets and feral animals alike, such as playgrounds, parks, sandboxes and dirt to reduce risk of accidentally ingesting Toxocara eggs.1,4 On a community level, laws, some known as “scoop laws” mandating timely removal of dog and cat feces should be enacted and enforced.4,14 On the national level, “control” programs addressing stray and feral dog populations have additionally been advocated to reduce transmission.14
Lastly, as consumption of uncooked, contaminated meat is a major source of toxocariasis in adults, education of target populations likely to consume these high risks products may be effective in reducing transmission of the disease.5
Differential Diagnosis
While toxocariasis can present with a multitude of clinical findings, it is important to consider additional ocular diagnoses to ensure appropriate treatment is enacted. One of the most important differential diagnoses to consider is retinoblastoma, as this is the most common intraocular cancer of childhood and can ultimately be fatal if not detected and managed early.39,40 Retinoblastoma can present with leukocoria most commonly, followed by strabismus.39,40 Other less frequent clinical signs and symptoms include decreased visual acuity and poor visual tracking, inflammation and proptosis.39,40
Other differentials include Coat’s disease, persistent hyperplastic primary vitreous (PHPV), retinopathy of prematurity (ROP) and congenital cataracts. Coat’s disease is described as an idiopathic retinal telangiectasia typically occurring unilaterally and within the first or second decade of life.41 Patients will often present with decreased visual acuity, leukocoria or strabismus due to retinal vascular anomalies that lead to leakage and exudation resulting in retinal detachment.41
PHPV is a congenital developmental anomaly noted in infants resulting from the failure of the structures of the primary vitreous to regress.42 These patients can present with leukocoria due to retrolental tissue, microphthalmia, intraocular hemorrhages, dense vitreal condensation and retinal folds.42,43 Similarly ROP presents in infancy, although this occurs as a result of incomplete retinal vascularization. Clinically this can present with neovascularization, retinal traction leading to retinal detachment, dragged vessels and retinal folds.44
Toxocara is ubiquitous with significant potential for long term detrimental effects to both ocular and systemic health. Awareness of the disease, its sequelae, effective treatment and management strategies, as well as prevention measures can be an important step in controlling this public health concern.
Dr. Gurwood thanks Drs. Leann Jaroczynski, Andrew Rixon for contributing this case.
1. Ahn SJ, Ryoo NK, Woo SJ. Ocular Toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pac Allergy. 2014;4(3):134-41. |

