 One of the fastest growing cancer diagnoses in the United States, esophageal adenocarcinoma is most commonly diagnosed in white males over age 65 with a history of obesity, gastroesophageal reflux disease, smoking and alcohol use.1 Gastrointestinal tumors, such as esophageal adenocarcinoma, often metastasize to the liver, bone, lung, adrenal glands and brain—less commonly to the eye and orbit.2
One of the fastest growing cancer diagnoses in the United States, esophageal adenocarcinoma is most commonly diagnosed in white males over age 65 with a history of obesity, gastroesophageal reflux disease, smoking and alcohol use.1 Gastrointestinal tumors, such as esophageal adenocarcinoma, often metastasize to the liver, bone, lung, adrenal glands and brain—less commonly to the eye and orbit.2
A literature review revealed six published cases of choroidal metastasis from primary esophageal adenocarcinoma.2-8 In the majority of these cases, the patient recently had been diagnosed with esophageal adenocarcinoma. Only one case involved the choroidal mass as the initial manifestation of the disease.
To the best of my knowledge, the case described in this report is the only published instance in which a choroidal metastasis was the presenting sign in a patient without ophthalmic or overt systemic symptoms at the time of detection.
History
A 63-year-old white male presented to the Wade Park Veterans Affairs Medical Center Optometry Clinic in Cleveland for a routine intraocular pressure check and dilated fundus exam (figure 1). He had no visual or asthenopic complaints and no known changes to his systemic health. His ocular history included plateau iris syndrome O.U., which was treated with laser iridoplasty 14 months earlier, and mild non-visually significant nuclear sclerosis.
1. Anterior surface of the patient’s right eye.
His medical history was significant for morbid obesity, hypertension, hyperlipidemia, steatohepatitis, benign prostatic hypertrophy, post-traumatic stress disorder and chronic pain. This patient also had a history of bone cancer—he had a malignant fibrous histiocytoma (a soft-tissue sarcoma) in his right leg, which was resected in 1994, without any subsequent recurrence or need for additional treatment.
Although the patient had abstained from tobacco for 25 years and from alcohol for about 40 years, his records indicated that he had previously smoked two packs of cigarettes and drank a fifth of liquor (750ml) per day (the length of time was unable to be determined from the patient’s chart).
The patient’s last eye exam was six months prior—due to his diagnosis of plateau iris syndrome, he had routine dilated fundus exams every six to 12 months. There was no evidence of any choroidal or retinal abnormalities at his last dilated fundus examination.
Diagnostic Workup and Differential Diagnoses
On examination, spectacle-corrected visual acuity was 20/20 O.U. Pupils, extraocular muscle testing and confrontation visual field testing were normal O.U. An automated Humphrey Matrix FDT (Carl Zeiss Meditec) 30-5 screening visual field test showed reliable and repeatable depression of one nasal edge point O.D., and a full-field O.S. Intraocular pressure was 16mm Hg O.U. The anterior segment biomicroscopy examination was remarkable for a patent laser peripheral iridotomy performed superiorly in each eye, and laser iridoplasty scars at the iris periphery 360° in each eye.
Dilated fundus exam of the left eye revealed 1+ nuclear sclerosis, a clear vitreous, a normal optic disc with a healthy neuroretinal rim, normal vasculature, and a normal macula and retinal periphery. The right eye revealed 1+ nuclear sclerosis, clear vitreous, normal vasculature and an optic disc with a healthy neuroretinal rim.
An elevated choroidal lesion, measuring approximately 6DD in size, was noted temporal to the macula (figures 2 and 3). The lesion appeared as two elevated focal nodes separated by a shallow valley. The lesion’s surface had a mottled yellowish appearance, distinct borders and adjacent retinal folds.
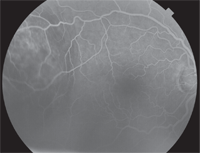
Spectralis HRA SD-OCT (Heidelberg Engineering) showed a >1,500µm choroidal elevation with an adjacent serous retinal detachment (figure 4). A fundus autofluorescence (FAF) scan of the lesion indicated overall hyperautofluorescence, punctuated by smaller zones of hypoautofluorescence (figure 5). Intravenous fluorescein angiography showed a mottled appearance with early hyperfluorescence that became more prominent later in the angiogram (figures 6 and 7).
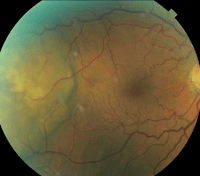
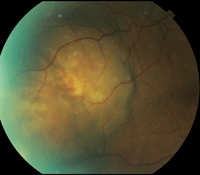
2, 3. Fundus images show the proximity of the choroidal lesion to the macula and its extent of involvement.
The differential diagnoses included primary choroidal melanoma and choroidal metastasis from a non-ocular primary tumor site. A thorough chart review of the patient’s previous dilated fundus exams revealed no evidence of a pre-existing choroidal nevus.
Clinical clues, such as the mottled yellowish coloration, serous retinal detachment and multiple foci, suggested that this lesion might be a choroidal metastasis from a non-ocular primary tumor site rather than a primary choroidal melanoma.
The retina specialist on staff reviewed the patient’s SD-OCT scan, autofluorescence and fluorescein angiography images, and examined him the same day. After discussing the urgency of the case, he and I directly contacted a staff oncologist who helped determine the next steps for this patient’s diagnosis. The oncologist suggested ordering a PET scan to determine if this was an isolated ocular lesion, or if it was a distant metastasis from another primary tumor site. The patient was scheduled for a PET scan 11 days later.
Unfortunately, 10 days later, one day prior to his scheduled testing, the patient presented to the emergency department with severe abdominal pain, diarrhea, dysphagia, night sweats without fever and an unintentional 10-pound weight loss over the past week. An abdominal CT scan revealed several concerns: thickening of the distal esophagus, an abnormal appearance of the liver suggestive of neoplasm, prominent adrenal glands suspicious for bilateral adrenal adenomas, and a possible focal mesenteric infarct. The patient was admitted for a malignancy workup.
An esophagogastroduodenoscopy was performed and was consistent with the CT scan results. The results were remarkable for Barrett’s esophagus that extended from a spot that was located 27cm from the entry point to the gastroesophageal junction located 39cm from the entry point (figure 8). Within this segment of Barrett’s esophagus (30cm from the entry point to the gastroesophageal junction), was a large, fungating, ulcerated mass (figure 9). A biopsy of the mass revealed invasive, moderately differentiated adenocarcinoma. Without any immediate need for surgical intervention, the patient was discharged and sent for an outpatient PET scan.
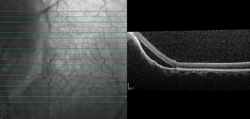
4. An SD-OCT line scan through the choroidal lesion reveals the presence of adjacent subretinal fluid.
The outpatient PET scan showed that the distal portion of the esophagus had marked hypermetabolic activity. An abnormally intense signal was observed throughout the liver, indicating lesions too numerous to count. Other significant areas were observed in the left adrenal gland, sternum, L1 vertebral body, iliac bones, sacrum, acetabulum, femur and nodules within the paraspinal and gluteal musculature.
Diagnosis
The radiologist’s report suggested a primary malignancy of the distal esophagus with likely metastasis, which was confirmed with an ultrasound-guided liver biopsy. The patient was diagnosed with rapidly progressing stage IV esophageal adenocarcinoma with liver, bone and choroidal metastasis. A choroidal biopsy was not performed because it is assumed that the mass was a true choroidal metastasis secondary to the widespread systemic involvement that was confirmed with PET scan and liver biopsy.
Within the next two weeks, the patient began to suffer from jaundice, liver failure, a right lower extremity deep vein thrombosis and uncontrolled pain with constant nausea and anorexia. The oncologist determined that the patient was not a candidate for treatment due to poor functional status. He was admitted into hospice for end-of-life care and passed away the following day.
Discussion
The incidence of esophageal cancer has skyrocketed in just a few decades—some estimates suggest that it has increased by more than 350% in the United States over the past 40 years.9 This rise has been attributed in part to the increased incidence of gastroesophageal reflux disease and Barrett’s esophagus due to the additive effects of smoking, drinking alcohol, poor diet and obesity.7,9 Esophageal cancer is most prevalent in white males over age 65, with risk factors that include obesity, gastroesophageal reflux disease and the subsequent development of Barrett’s esophagus.9
Barrett’s esophagus is defined by metaplasia of the normal stratified squamous epithelium that lines the distal portion of the esophagus with replacement tissue (growth of abnormal columnar epithelium).9 These abnormal columnar cells are at risk for dysplasia, in which DNA mutations cause these cells to hyperproliferate. During this process, additional DNA mutations and changes in cell morphology lead to neoplastic cell growth.10 Approximately 5% to 15% of individuals with gastroesophageal reflux disease have evidence of Barrett’s esophagus, and less than 1% of them develop cancer each year.11
Smoking and alcohol use also are well-documented risk factors for esophageal adenocarcinoma. In this case, the patient was morbidly obese, had a history of gastroesophageal reflux disease with presence of Barrett’s esophagus, and reported a remote––but significant––history of tobacco and alcohol use.
Metastasis
While the diagnostic rate of esophageal cancer is increasing in the United States, it still remains a rare type of cancer. Metastasis of a primary gastrointestinal tumor to the eye is even more rare. It occurs through hematogenous dissemination. The choroid is very richly vascularized by posterior ciliary artery circulation, which makes it the most common site for intraocular metastases.12 In fact, metastatic disease to the choroid is the most common ocular malignancy.12
Choroidal metastases are bilateral in 20% to 40% of cases, and about 20% are multifocal within one eye.12 In a survey of more than 500 eyes with uveal metastases, 88% involved the choroid, while the remaining cases involved the iris and/or ciliary body.13 These choroidal metastases are most often from primary breast cancer in women and from lung cancer in men.13 Other primary tumors that may metastasize to the choroid include those of the prostate, kidney, thyroid, pancreas and gastrointestinal sites.13,14 The presence of choroidal metastasis suggests a poor prognosis, as it indicates the likelihood of additional metastases.
Distinguishing Characteristics

5. A fundus autofluorescence scan showed overall
hyperautofluorescence, with scattered areas of hypo- autofluorescence
throughout the eye.
The differential diagnosis of an acute presentation of an elevated choroidal mass should include life- and sight-threatening conditions first, such as primary ocular malignancy and ocular metastasis from a non-ocular primary tumor. These should be followed by more benign conditions, such as choroidal osteoma, choroidal detachment and choroidal hemangioma.
- Yellow coloration with retinal pigment epithelium (RPE) changes.
- Plateau shape of 3mm or greater in thickness.
- Associated subretinal fluid.
For this patient, we performed an SD-OCT scan, FAF and intravenous fluorescein angiography (IVFA) to distinguish these characteristics. B-scan ultrasound might have been useful in this case, but it was not performed at the initial visit and the patient did not return for follow-up. An A-scan might be considered for size determination, and might also give additional information about the vascularity of the lesion.
Diagnostic Imaging
• SD-OCT. The SD-OCT scan helped to elicit information about the height and thickness of the mass and to identify any associated subretinal fluid.The presence of subretinal fluid is secondary to increased choroidal vascular permeability as well as damaged RPE cells that can no longer expel accumulating fluid. In cases where visual acuity is preserved, the subretinal fluid is too far from the macula to induce visually significant changes.
However, in these cases patients become symptomatic consistent with tumor growth and expansion of the subretinal fluid into the macula. Symptoms generally include blurred vision, metamorphopsia, increased IOP, pain from impingement of ciliary nerves, photopsia and/or floaters due to the retinal disruption.2-8
• FAF. FAF may be used as a marker for retinal disease due to the pathological accumulation of naturally occurring lipopigments.15 One such lipopigment, lipofuscin, can autofluoresce and spontaneously emit radiance when illuminated with light of a different wavelength.
Lipofuscin accumulates in the RPE when the cells’ metabolic machinery is functioning abnormally. The accumulation of lipofuscin is exhibited as hyperautofluorescence, which appears bright white on the scan, whereas photoreceptor or RPE loss is shown as hypoautofluorescence and appears dark on the scan. This pattern of autofluorescence suggests substantial damage to the RPE and photoreceptor layers in an area corresponding with a lesion.
• IVFA. Intravenous fluorescein angiography detects abnormalities in retinal circulation. In the literature––malignant lesions are marked by the mottled appearance of the lesion with increasing hyperfluorescence throughout the angiogram corresponding to localized RPE disruption, increased vascular permeability and subretinal fluid.7 Late in the angiogram, the presence of small, well-defined, hyperfluorescent foci become evident within the lesion. These likely correspond to hyperpermeable blood vessels within the tumor itself.7
|
 |
| 6, 7. Intravenous fluorescein angiogram images show localized RPE disruption, increased vascular permeability and subretinal fluid (venous phase, left) as well as small, well-defined, hyperfluorescent foci that likely correspond to hyperpermeable blood vessels within the tumor itself (late venous phase, right).
| |
Without systemic symptoms or a recent history of a known malignant tumor, it was difficult to determine if this patient’s lesion was a primary ocular tumor or a metastatic lesion caused by a distant non-ocular tumor. In the literature, five of the six documented cases that differentiate choroidal metastasis from esophageal adenocarcinoma occurred in individuals with a recent pre-existing cancer diagnosis.2,4-8 Only one case involved a choroidal metastasis as the initial presentation of esophageal adenocarcinoma.3 In that instance, the patient had a two-month history of decreased vision in the affected eye as well as a two-month history of dysphagia, which is the most common symptom of esophageal adenocarcinoma.16
To our knowledge, the patient in this case did not have any systemic symptoms at the time of his eye exam, and furthermore did not have a recent history of cancer diagnosis. The PET scan was ordered to help differentiate between isolated ocular vs. widespread metastatic disease. It suggested a primary neoplasm at the distal esophagus with widespread metastasis to the liver, bone, adrenal glands and several lymph nodes.
Metastasis was later confirmed with ultrasound-guided liver biopsy.
Prognosis and Treatment
With an average five-year survival rate of less than 17%, esophageal adenocarcinoma has a very poor prognosis––especially when the disease is metastatic.1,9,17-19 The majority of patients have systemic metastasis at the time of diagnosis. Even with treatment, the median survival of metastatic esophageal adenocarcinoma is less than one year.17
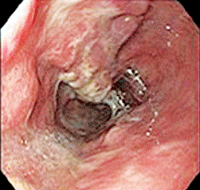

8, 9. An esophagogastroduodenoscopy revealed Barrett’s esophagus (left). A large, fungating, ulcerated mass also was found within this section of Barrett’s esophagus. Biopsy revealed that it was invasive adenocarcinoma.
Systemic treatment options include surgical resection, chemotherapy, radiation and improving or maintaining quality of life.1,9,17,18 Treatment options for the choroidal metastasis itself include chemotherapy, local irradiation and enucleation.2-8
Most recently, experimental use of intravitreal anti-VEGF therapy for choroidal metastases from lung, breast and colorectal cancer has shown good results with significant tumor regression.20
This unique case illustrates the importance of routine dilated fundus exams for a patient’s systemic health. Optometrists should be familiar with the common characteristics of chorioretinal lesions and be able to use and interpret specialized diagnostic testing in order to finalize a proper differential diagnosis.
The fundus findings in this case pointed toward extensive systemic disease that had advanced beyond the point of feasible intervention. However, early detection of this devastating cancer may help increase longevity and quality of life for individuals without such advanced disease.
Dr. Schonberg saw this patient during her ocular disease residency at the Wade Park Veterans Affairs Medical Center Optometry Clinic in Cleveland.She now works as an optometrist at Lasik Plus and Visium Eye Institute, in Independence, Ohio. She would like to thank staff optometrists Stacia Yaniglos, O.D., and Terry Daniel, O.D., and retinal specialist Eric Eleff, M.D., at the Louis Stokes VA Medical Center in Cleveland.
1. Sohal D, Sun W, Haller D. Pancreatic, gastric, and other gastrointestintal cancers. ACP Medicine. May 2011. Available at:
www.acpmedicine.com. Accessed September 25, 2012.
2. Knezevi J, Radovanovi N, Simi A, et al. Isolated choroidal metastasis from primary adenocarcinoma of the distal esophagus: a case report. Dis Esophagus. 2003;16(1):41-3.
3. Eliott D, Salehi-Had H, Plous OZ. Adenocarcinoma of the esophagus presenting as choroidal metastasis. Dis Esophagus. 2011 Feb;24(2):E16-8.
4. Buskens CJ, Tan HS, Hulscher JB, et al. Adenocarcinoma of the esophagus with choroidal metastasis. Dis Esophagus. 2001;14(1):70-2.
5. Parikh HK, Deshpande RK, Swaroop DV, Desai PB. Choroidal metastasis from primary adenocarcinoma of the esophagus. J Surg Oncol. 1993 Jan;52(1):68-70.
6. Hegde SR, Wagle AM, Au Eong K. Retinal detachment as a presenting feature of metastatic esophageal adenocarcinoma. Am J Gastroenterol. 2010 Feb’105(2)474-6.
7. Samuel J, Flood TP, Agbemazdo B, et al. Choroidal metastasis from adenocarcinoma of the esophagus. Retina. 2003 Dec;23(6):874-7.
8. Singh D, Sharma A, Arora B, et al. Adenocarcinoma esophagus with choroid metastasis. Indian J Gastroenterol. 2004 May-Jun;23(3):112-3.
9. Esophageal and gastroesophageal junction cancers. In: Kantarjian HM, Wolff RA, Koller CA. The MD Anderson manual of medical oncology. 2nd ed. New York, NY: McGraw-Hill. 2011.
10. Gastric and esophageal cancer. In: Kantarjian HM, Wolff RA, Koller CA. The MD Anderson manual of medical oncology. 2nd ed. New York, NY: McGraw-Hill. 2011.
11. Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002 Apr 17;287(15):1972-81.
12. Ryan SJ (ed). Retina 2nd ed. Philadelphia: Mosby Inc. 1994.
13. Shields CL, Shields JA, Gross NE, et al. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997 Aug;104(8):1265-76.
14. Steidl SM, Hartnett ME. Clinical pathways in vitreoretinal disease. New York: Thieme Medical Publishers, Inc. 2003.
15. Slotnick S, Sherman J. Panoramic autofluorescence: highlighting retinal pathology. Optom Vis Sci. 2012 May; 89(5):E575-84.
16. Herbella FAM, Patti MG. Esophageal cancer clinical presentation. Medscape Reference. Available at: http://emedicine.medscape.com/article/277930-clinical. July 10, 2012. Accessed October 1, 2012.
17. Cassel CK, Leipzig RM, Cohen HJ, et al (eds). Geriatric medicine: An evidence-based approach. 4th ed. New York: Springer-Verlag; 2003.
18. The neoplastic esophagus. In: Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, et al. Gastrointestinal pathology, an atlas and text. 3rd ed. Philadelphia: Lippincott Williams & Wilkins. 2008.
19. Cummings LC, Cooper GS. Descriptive epidemiology of esophageal carcinoma in the Ohio Cancer Registry. Cancer Detect Prev. 2008;32(1):87-92.
20. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastasis. Surv Ophthalmol. 2011 Nov-Dec;56(6):511-21

