 History
History
A 52-year-old black female presented complaining of severely blurred vision in her left eye that had persisted for two months with no improvement. She reported no significant ocular or systemic history, and she denied using any medications.
Diagnostic Data
Uncorrected visual acuity measured 20/20 O.D. and 20/200 O.S. There was no afferent pupillary defect O.S. Confrontational visual fields uncovered a central area of blur. Amsler grid testing confirmed this finding and demonstrated a small, central relative scotoma. Color and brightness testing were also slightly reduced O.S. Refraction uncovered negligible error, with clear retinoscopic reflexes. The OD demonstrated unremarkable findings.
Biomicroscopy revealed normal anterior segment structures and normal anterior chambers both eyes. The patients intraocular pressure measured 12mm Hg, OU. The pertinent finding was uncovered on dilated fundus examination and is illustrated in the photograph.
Additional tests included the WatzkeAllen test, which demonstrated a broken, distorted streak of light O.S. when placed over the macula. Other useful tests may include optical coherence tomography, focused macular perimetry and photodocumentation.
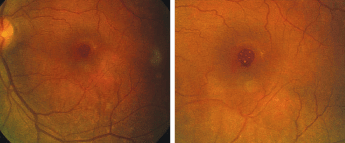
The left eye of our 52-year-old patient with blurred vision. Close-up (right).
Your Diagnosis
How would you approach this case? Does this patient require any additional tests? What is your diagnosis? How would you manage this patient? Whats the likely prognosis?
The Diagnosis
This patient has a stage IV macular hole. Idiopathic, age-related macular holes (IMHs) are one cause of severely reduced central vision.1-7 Women past age 65 are most commonly affected.1,4,6,8-14
Symptoms of an impending macular hole include decreased vision and metamorphopsia. The current standard of treatment for early, full-thickness macular holes (FTMH) is surgerynot observation. Surgical procedures have been shown to decrease the incidence of hole enlargement and can result in improved visual acuity.1,2
Most macular holes are unilateral, with a reported incidence of bilateral presentation between 0% and 29%.4 Risk factors for the development of macular hole in the fellow eye include pigment epithelial defects and macular retinal thinning.9 The presence of a posterior vitreous detachment in the unaffected eye lowers the risk to almost 0%.9, 22
The fovea does not contain the inner plexiform, ganglion cell or nerve fiber layers that are present in the rest of the retina.15 These elements are diverted laterally. So, the fovea is thinnest in the center and is thickest at its margins.
Average foveal concavity is 1,500m in diameter. The central 500m of the fovea is free of rod photoreceptors and capillaries. Blood supply to most of the fovea occurs via the capillary branches of the central retinal artery, while the central avascular zone receives its blood supply from the choriocapillaris.8
The vitreous maintains a close relationship with the macula. The vitreous is composed of a random framework of type II collagen fibers and hyaluronic acid. The outer portion of the vitreous, the cortex, contains more collagen fibers and lies along the retinal surface. The vitreous has firm attachments to the retina in the area of the vitreous base (the peripapillary region), areas overlying major retinal vessels and the area surrounding the fovea. Vitreous movement over these areas of attachment likely plays a role in the pathogenesis of IMH.8
Causes of macular holes include trauma, vitreous-related degeneration, solar retinopathy and degenerative/pathologic myopia.9,10 The majority of macular holes are both age-related and idiopathic in nature.8,12,16 IMHs usually affect older patients (ages 60 to 70).1,9
The etiology of an IMH has not been conclusively identified.9 Cystoid macular edema, choroidal vascular insufficiency and anteroposterior vitreoretinal traction have been implicated.9, 12,14 Recently, theories of tangential vitreoretinal traction have gained acceptance as a potential mechanism for an IMH.1,3,8-10,12,17
The late J. Donald M. Gass, M.D., devised the widely accepted method of classifying macular holes. He separated the progression of macular holes into four stages:
Stage I (macular cyst). A stage I macular hole is defined by a serous detachment of the fovea. In early stage I holes (stage I-A), the concavity of the fovea is lost, and a yellow spot that represents increased visibility of the xanthophyll pigment appears in the center of the macular area. Later (stage I-B), the pigment is displaced in an outward fashion, as it migrates toward the outer regions of the foveas circumference. The impending hole produces a pigmented ring-shaped lesion.8,13,18 This change in pigment appearance, from a spot to a ring, is unique to macular hole development. In 50% of cases, the process is spontaneously aborted.18
Stage II (early macular hole). A stage II macular hole is defined by full-thickness retinal dehiscence. On biomicroscopy, it appears as an oval-, crescent- or horseshoe-shaped retinal defect on the inside edge of the xanthophyll ring. It can emerge as a central, round retinal defect that is surrounded by a rim of elevated retina, either with or without an overlying prefoveal opacity. These macular holes may enlarge secondary to centrifugal movement of retinal receptors.18 Up to 70 % of stage II holes progress to stage III.9
Stage III (full-thickness macular hole). Stage III is defined by a hole from 400m to 600m in diameter that is surrounded by a rim of elevated retina. Here, the vitreous becomes separated from the fovea, and a prefoveal opacity, which represents this separation, may or may not be visible. Posterior vitreous detachment (PVD) is not present in stage III holes.8,18
Stage IV (FTMH). Stage IV macular holes demonstrate a detachment of the posterior vitreous. A pseudo-operculum (Weiss ring), if present, is usually found near the temporal border.
Patients with stage I and II macular holes present with symptoms of decreased visual acuity/metamorphopsia; individuals with stage III macular holes demonstrate significantly more depressed acuity.9-11 Visual acuity for eyes with an FTMH ranges from 20/40 to 5/200, with an average of 20/200.8 Central scotomas that correspond to the macular hole can be confirmed using a scanning laser ophthalmoscope and focused perimetry.7,8
Fluorescein studies are usually normal in cases associated with impending macular holes. Early hyperfluorescence has occasionally been reported in cases of late stage I lesions.8,14 This phenomenon is thought to be secondary to the absence of xanthophyll pigment.8 Fluorescein angiography of macular holes between stages II and IV typically reveals an area of early hyperfluorescence.8,9,14,18
Prompt identification of macular holes is critical for treatment.7,9 The differential diagnoses include pseudoholes (vitreoretinal interface abnormalities), epiretinal membranes (proliferation of glial cells through a break in the internal limiting membrane), lamellar holes (non-full-thickness breaks), cystoid macular edema, age-related macular degeneration and vitreomacular traction syndrome.11
Visual acuity and visual status (integrity, as well as field) are also important clues. Carefully examine the macular area using binocular indirect biomicroscopy. Be sure to study the posterior vitreous for evidence of vitreofoveal separation and pseudo-operculum.
Also, perform a slit-beam test when looking for the Watzke-Allen sign. Focus a slit beam on the macula so that its width fits within the boundaries of the presumed hole. The test is positive if the patient reports a broken line.11
Fluorescein angiography is not considered a mandatory diagnostic test, as there have been reports of FTMHs that do not possess hyperfluorescence.19 One study recently demonstrated the successful application of a scanning laser ophthalmoscope to distinguish full-thickness macular holes from other similar conditions, such as variations of an impending macular hole.7
The prognosis for idiopathic macular holes is fair. Only 50% of stage I macular holes progress to stage II, but 70% of holes that reach stage II progress to stage III.7-9
Neil Kelly, M.D., and Robert Wendel, M.D., were the first to use pars plana vitrectomy and gas tamponade followed by one or more weeks of face-down positioning to achieve anatomical hole closure.5,6,21 Visual acuity improved by two or more lines in 42% of patients.21 Since then, studies have been conducted to examine the benefit of vitrectomy surgery for macular holes.2,4,23 Results of multicentered, randomized clinical trials show no benefit of surgery over observation for stage I macular holes.2,4,23
A multicenter, randomized clinical trial conducted by the Vitrectomy for Macular Hole Study Group found that pars plana vitrectomy in patients with stage II macular holes decreased the incidence of progression to stages III and IV, with better acuity compared to observation alone.2 Another study found that patients who had pars plana vitrectomy performed within two months of the onset of symptoms fared best.23 A randomized clinical study to investigate the benefit of surgery for stage III and IV macular holes has not yet been conducted.2
Other studies have investigated the use of adjunctive agents and removal of the internal limiting membrane during vitreomacular procedures to improve anatomic hole closure and acuity recovery. One study used thrombin-activated fibrinogen and selective removal of the internal limiting membrane to achieve closure in up to 96% of cases.26 The use of chemical adjuvants, while not proven conclusively, shows promise and is worthy of clinical study.
Stage I holes should be observed for progression. Stage II holes should be treated promptly by a retinologist who is experienced in vitreomacular procedures. Current modalities include pars plana vitrectomy with gas tamponade using SF6 (sulfahexafluoride) or C3F8 (perfluoropropane) with subsequent 80% to 90% face-down positioning for two weeks. Classic complications following surgery include cataract formation, retinal pigment epithelium alterations, retinal breaks or detachment, hole enlargement, hole reopening, vascular occlusion, cystoid macular edema, choroidal neovascularization, field loss and endophthalmitis.3,24,25 Stage III holes do not appear to benefit from surgery. Patient education and low vision rehabilitation offer potential assistance.
We referred our patient to a retinal specialist, who confirmed the diagnosis and offered a surgical solution. The patient has yet to commit to the procedure and has not yet seen any spontaneous improvement.
Thanks to Karen Jones, O.D., of
1. Gregor ZJ. Surgery for idiopathic full-thickness macular holes. Eye 1996;10(Pt 6):685-90. Review.
2. Kim JW, Freeman WR, Azen SP, et al. Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Vitrectomy for Macular Hole Study Group. Am J Ophthalmol 1996 Jun;121(6):605-14.
3. Banker AS, Freeman WR, Kim JW, et al. Vision-threatening complications of surgery for full-thickness macular holes. Ophthalmology 1997 Sep;104(9):1442-52; discussion 1452-3.
4. Ezra E, Wells JA, Gray RH, et al. Incidence of idiopathic full-thickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology 1998 Feb;105(2):353-9.
5. Nao-i, N. Pearls in the management of macular holes. Semin Ophthalmol 1998 Mar;13(1):10-9.
6. Minihan M, Goggin M, Cleary PE. Surgical Management of macular holes: results using gas tamponade alone, or in combination with autologous platelet concentrat, or transforming growth factor b2. Br J Ophthalmol 1997 Dec;81(12):1073-9.
7. Tsujikawa M, Ohji M, Fujikado T, et al. Differentiating full thickness macular holes from impending macular holes and macular pseudoholes. Br J Ophthalmol 1997 Feb;81(2):117-22.
8. Gass JD. Macular dysfunction caused by vitreous and vitreoretinal interface abnormalities. In: Gass JD (ed). Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment.
9. Alexander LJ. Exudative and nonexudative macular disorders. In: Alexander LJ (ed). Primary Care of the Posterior Segment.
10. Kanski JJ. Degenerations and dystrophies of the fundus. In: Kanski JJ (ed). Clinical Ophthalmology: a Systematic Approach, 3rd ed.
11. Margherio AR, Margherio RR. Macular holes and epiretinal macular membranes. In: Tasman W and Jaeger EA (eds). Duane"s Clinical Ophthalmology.
12. Benson WE. Surgery for Macular Holes. In: Laibson PR (ed). The Year Book of Ophthalmology 1992.
13. Gass JD. Idiopathic senile macular hole: its early stages and pathogenesis. Arch Ophthalmol 1988 May;106(5):629-39.
14. McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol 1982 Jun;93(6):777-86.
15. McDonnell JM. Ocular embryology and anatomy. In: Ryan S (ed). Retina.
16. Aaberg TM, Blair CJ, Gass JD. Macular holes. Am J Ophthalmol. 1970 Apr;69(4):555-62.
17.Akiba J, Ishiko S, Hikichi T, et al. Imaging of Epiretinal Membranes in Macular Holes by Scanning Laser Ophthalmoscopy. Am J Ophthalmol 1996 Feb;121(2):177-80.
18. Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 1995 Jun;119(6):752-9.
19. Lewis H, Cowan GH, Straatsma BR. Apparent disappearance of a macular hole associated with development of an epiretinal membrane. Am J Ophthalmol 1986 Aug 15;102(2):172-5.
20. Lewis ML, Cohen SM, Smiddy WE, Gass JD. Bilateraly of idiopathc macular holes. Graefes Arch Clin Exp Ophthalmol 1996 Apr;234(4):241-5.
21. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 1991 May;109(5):654-9.
22. De Bustros, S. Vitrectomy for prevention of macular holes: results of a randomized multicentered clinical trial. Ophthalmology 1994 Jun;101(6):1055-9; discussion 1060.
23. Willis AW, Garcia-Cosio JF. Macular hole surgery. Comparison of longstanging versus recent macular holes. Ophthalmology 1996 Nov;103(11):1811-4.
24. Paques M, Massin P, Santiago PY, et al. Late reopening of successfully treated macular holes. Br J Ophthalmol 1997 Aug;81(8):658-62.
25.Hutton WL, Fuller DG, Snyder WB, et al. Visual field defects after macular holes surgery. A new finding. Ophthalmology 1996 Dec;103(12):2152-8; discussion 2158-9.
26. Olsen TW, Sternberg P Jr, Capone A Jr, et al. Macular hole surgery using thrombin-activated fibrinogen and selective removal of the internal limiting membrane. Retina 1998;18(4):322-9.

