Annual Cornea ReportCheck out these articles: The Many Layers of Cornea Comanagement |
It is unusual for a treatment algorithm to be dramatically re-written for ophthalmic pathology. This occurred in the early 2000s with anti-VEGF medications taking over as the treatment of choice for wet macular degeneration and subsequently most retinal vascular issues. At the same time, posterior lamellar transplants began replacing penetrating keratoplasty (PK) as the surgical treatment of choice for all forms of endothelial decompensation. We may currently be in the initial stages of another such shift in the clinical management of corneal endothelial disease with the use of rho kinase (ROCK) inhibitors.
A relatively new ophthalmic class, ROCK inhibitors have been around for a few years in the form of Rhopressa (netarsudil, Aerie) and Rocklatan (netarsudil/latanoprost, Aerie) in the field of glaucoma. The revelation that a glaucoma med might be repurposed for an altogether different condition involving an unrelated ocular structure could, if validated by large-scale studies, emerge as one of this decade’s biggest success stories.
Grow Your Own
The mechanism of ROCK inhibitors for IOP control has to do with the molecules’ influence on cytoskeleton and intracellular adhesions. These cytostructural changes result in a decrease in resistance to aqueous outflow at the point of the trabecular meshwork. Beyond the specific influence of ROCK inhibition, Rhopressa also targets norepinephrine transport, which secondarily reduces production of aqueous and reduced IOP.1,2
The two netarsudil-containing medications offer a nice, once-daily adjunct to the medical management of glaucoma and, depending on out-of-pocket patient expense, are near the front of the therapeutic options here. However, evidence is continuing to mount that the role of ROCK inhibitors in the treatment of endothelial-based corneal edema could be even more profound.
Given that the corneal endothelium is arrested in the cell cycle and therefore not mitotic, any process that damages these cells can only be treated via transplanting new ones into the eye. Currently, the standard practice with any form of endothelial decompensation resulting in corneal edema—regardless of specific etiology—is essentially a period of “handholding” until the patient is so bothered by reduced vision that they require surgical intervention.
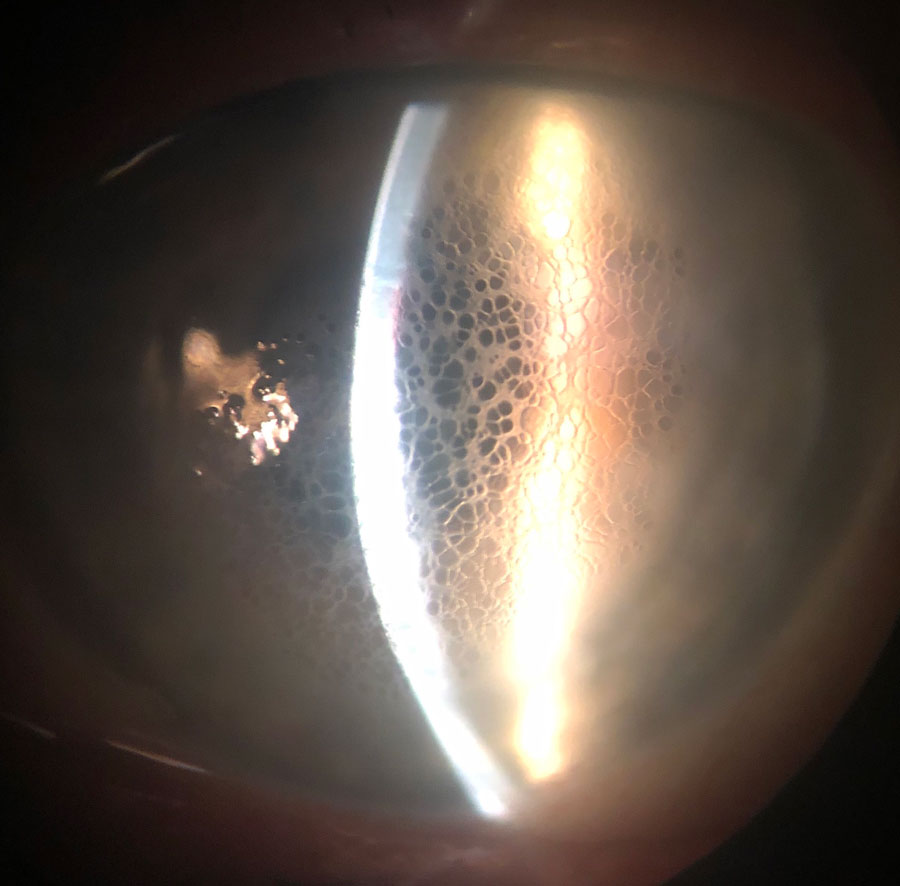 |
Diffuse honeycomb corneal edema in a post-DSAEK eye. This patient is taking Rhopressa once daily. Click image to enlarge. |
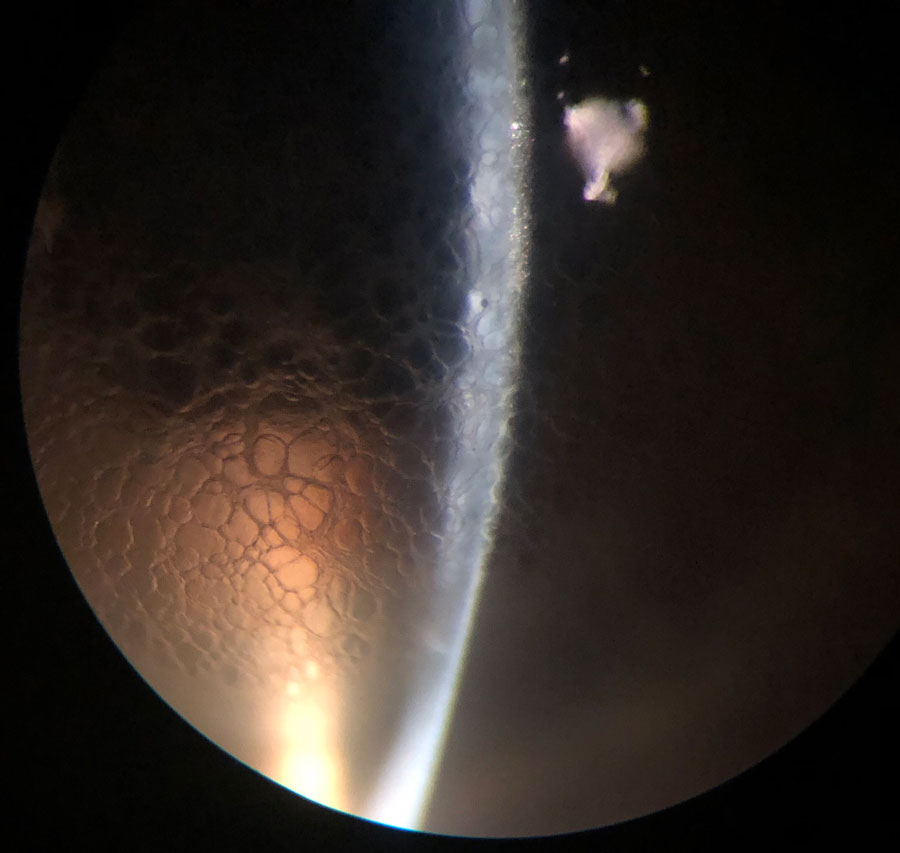
|
|
Optic section of same patient. Note the honeycomb is confined to the epithelial layer. Click image to enlarge. |
Though the threshold to consider transplant surgery for endothelial decompensation has dropped significantly since the advent and widespread adoption of lamellar procedures like Descemet’s stripping automated endothelial keratoplasty (DSAEK) and Descemet’s membrane endothelial keratoplasty (DMEK), these techniques still present significant hardships for patients. These may include:
• potential long travel to a surgery center for those who do not live near a surgeon
• the need for the patient to remain in a supine position the first few days postoperatively
• the possibility of graft detachment and need for a subsequent re-bubble
• ongoing risk of transplant rejection
• the potential sequelae of topical corticosteroid use needed to reduce risk of rejection
• failure of the graft over time and the need to re-transplant, as these grafts have a finite life expectancy
The ability to avoid or at least postpone these transplant-based issues led to the development of the Descemet’s stripping-only (DSO) procedure, also known as Descemetorhexis without endothelial keratoplasty (DWEK). In DSO, patients with substantial central and localized Fuchs’ dystrophy have the central zone of endothelium and Descemet’s membrane removed and then are left to allow their normal peripheral endothelial cells to migrate in and fill this zone without the light-scattering effects of guttata.
While DSO avoids many of the issues that develop with transplants, it is a niche surgery and only reasonable for a select population of patients with endothelial disease. In reality, it is likely more of a temporizing measure, as removing diseased endothelium does nothing to avoid long-term decompensation. Further, issues such as inducement of irregular astigmatism, deep scarring and non-clearing corneal edema, may also develop.3
Enter ROCK inhibitors. The mechanism of ROCK inhibition on cellular and intracellular adhesions is not limited to the trabecular meshwork. Within the cornea, these molecules seem to promote migration and spread of corneal endothelium, which perhaps enhances the normal spread of endothelium following injury and localized cellular loss. It also prevents cellular apoptosis, a laudable effect that is critical for preserving existing endothelium. This can be initiated in Fuchs’ dystrophy secondary to the physical strain placed on the cell walls of endothelium adjacent to guttata.1,2,4
Most exciting of all, there is some evidence that ROCK inhibitors may promote endothelial proliferation, which given the non-mitotic status of these cells, has enormous clinical potential.2,4-6 If ROCK inhibition truly does promote controlled proliferation of endothelium, the algorithm for the management of endothelium-mediated corneal edema may be re-written. These agents can also serve as important components of effective cultivation methods to propagate endothelial cells for use in cell-based therapies.
To date, the use of ROCK inhibition in corneal disease has been more complementary than revolutionary. Its first broad use in corneal disease was as adjunctive therapy to speed and facilitate recovery within DSO patients. More recently, as clinicians have become more comfortable with the use of these agents for corneal disease, case reports of ROCK inhibition prior to corneal surgery have been published. One small series in particular demonstrated the effect of ROCK inhibition in corneal edema secondary to a broad range of pathologies. The author of this series was quick to point out that not all patients with corneal edema will respond positively to ROCK inhibition, and even includes a case of treatment failure to illustrate the point—it can be impossible to predict at this point who will and won’t respond. Notably, this case series only had a treatment duration of a month, and its effect, when successful, was maintained even after cessation of netarsudil.7
All that said, the use of ROCK inhibitors for corneal endothelial disease is a subfield in its infancy. It is possible further research will show less efficacy than hoped for and its use may remain a purely secondary therapy—promoting both graft survival or recovery from DSO. However, it’s also possible that as we become better at predicting the effectiveness of the agents or we develop more effective and targeted agents, ROCK inhibition may periodically or even widely replace corneal transplantation. Regardless, we are standing on the precipice in the management of corneal endothelial disease. Time will tell where we go from here, but for now, it’s worth keeping a close eye on the research.
A New Clinical Entity
Regardless of how far the application of ROCK inhibitors goes in corneal endothelial disease, we are left with one lasting effect of the drug: the potential of these meds to produce a totally new clinical manifestation called honeycomb corneal edema, alternately known as both macrocystic epithelial edema and reticular bullous epithelial edema.
Honeycomb edema is characterized by profoundly diffused but clearly delineated “cells” of epithelial edema that makes it wildly different in appearance from typical microcystic epithelial edema. This fascinating entity is related entirely to the use of ROCK inhibition in eyes with stromal edema or compromised endothelial health and is extremely common among that narrow population, but should not occur in eyes with healthy corneas using the medication for glaucoma.
Its mechanism likely has nothing to do with the endothelial effects of the drug—as our own experience with the medication suggests—where we have seen eyes develop honeycomb edema despite significant reduction in corneal thickness. Rather, honeycomb edema is probably a manifestation of the ROCK inhibitor–induced changes in how the epithelium responds to the strain of corneal edema through alterations in its intracellular adhesions.
 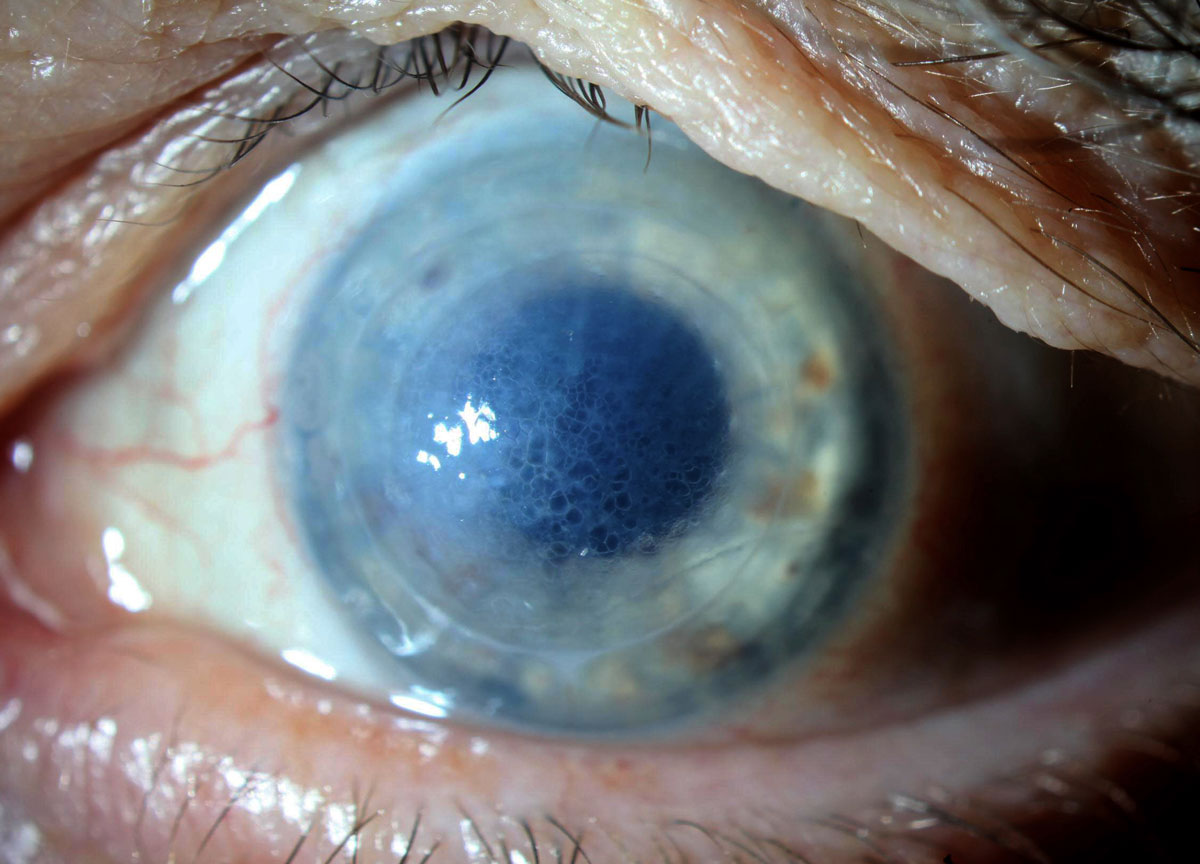
|
|
Honeycomb edema in a patient using off-label Rhopressa once daily as a medical tamponade in a failed DSAEK under PK. Click image to enlarge. |
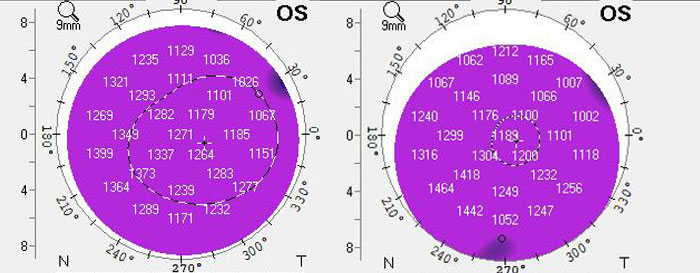 |
These are pachymetries before and after initiation of Rhopressa for the patient. Despite the development of honeycomb edema, the pachymetries are actually thinner following addition of Rhopressa. Further medical treatment with Rhopressa is ongoing. Click image to enlarge. |
It has been well documented that ROCK inhibitors increase the permeability of tight junctions.8 Tight junctions in the corneal epithelium promote maintenance of transparency by impairing passage of sodium ions and subsequent fluid into epithelial cells. Perhaps the honeycomb-shaped epithelial edema phenomenon is a result of ROCK inhibitors weakening corneal epithelial tight junctions, leading to an increased passage of fluid into these squamous, polygonal-shaped basal and surface epithelial cells in the setting of stromal edema—resulting in a clinical appearance resembling a honeycomb.
However, this is all still a theory and speculative, as cellular studies need to be conducted. According to the review cited earlier, all patients with stromal edema on ROCK-inhibitor therapy will develop honeycomb edema, and importantly, its development does not portend long-term treatment success or failure.8 In eyes that respond positively to the drug, the honeycomb edema will fade over time. In those that fail to respond to ROCK inhibition, edema will persist until the medication is discontinued at which point it will clear..2,8
Watch This Space
Though much more robust research is required prior to declaring ROCK inhibitors a front-line therapy in the clinical management of corneal edema, their use does hold promise even though this field is in its infancy. It’s also not every day a brand new clinical entity manifests, which is what ROCK inhibitors have created with honeycomb epithelial edema in patients with pre-existing stromal edema. While much can change with further research, its apparent we should all keep a close eye on the research with ROCK inhibition in corneal edema and make ourselves familiar with the complication, however benign, of its use in this population—honeycomb epithelial edema.
Dr. Bronner is an attending optometrist at Pacific Cataract and Laser Institute in Kennewick, WA. Dr. Do completed his residency in ocular disease and surgical comanagement at Georgia Eye Partners in Atlanta. He currently practices at Kaiser Permanente Northwest in Portland, OR. He is a Fellow of the American Academy of Optometry. They have no financial interests to disclose.
1. Moshifar M, Parker L, Birdsong OC, et al. Use of rho kinase inhibitors in ophthalmology: a review of the literature. Med Hypothesis Discov Innov Ophthalmol. 2018;7:101-11. 2. Syed Za, Rapuano CJ. Rho kinase (ROCK) inhibitors in the management of corneal endothelial disease Curr Opin Ophthalmol. 2021;31. 3. Davies E, Jurkunas U, Pineda R. Predictive factors for corneal clearance after descemetorhexis without endothelial keratoplasty. Cornea. 2018;37(2):137-140. 4. Kinoshita S, Colby K, Kruse FE. A close look at the clinical efficacy of rho-associated protein kinase inhibitor eye drops for Fuchs endothelial corneal dystrophy. Cornea. 2021. 5. Okumura N, Koizumi N, Kay EP, et al. The rock inhibitor eye drop accelerates corneal endothelial wound healing. Invest Ophthalmol Vis Sci. 2013; 54:2493-2502. 6. Koizumi N, Okumura N, Ueno M, et al. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea. 2013; 32:1167-70. 7. Davies E. Case series: novel utilization of rho-kinase inhibitor for the treatment of corneal edema. Cornea. 2021; 40:116-20. 8. Yin J, Yu FX. Rho kinases regulate corneal epithelial wound healing. AM J Physiol Cell Physiol 2008;295:C378-87. |

