
Not long ago, the only way to obtain a cross-sectional view of the retina or macula was with a histologic section. Although this offered a suitable way to study the macula of cadaver eyes, it was not practical for examining patients in a clinical setting.
With the advent of optical coherence tomography (OCT), however, we can now obtain this cross-sectional view of the macula in a clinical settingapproaching the resolution of excisional biopsy.1 OCT scans provide a reliable, objective, repeatable and painless way to obtain information about retinal pathology.
The potential for OCT was known more than 10 years agonamely, its ability to detect and monitor various macular diseases, including macular edema, macular holes, and detachments of the neurosensory retina and pigment epitheliumbut its true potential is only now being realized.2
New Technology
A few years ago, conventional time domain OCT (TD-OCT) technology was only available in teaching institutions, research centers, and other secondary and tertiary referral centers.
Until OCT became commercially available, fluorescein angiography (FA) was the gold standard for retinal imaging. However, FA requires injection of dye and has the potential for complications, such as anaphylaxis. OCT alone cannot match some qualities of FA, such as the ability to look at retinal perfusion and overall hemodynamics. For this reason, FA will continue to exist, but now as an adjunctive test to OCT. Since the Stratus OCT (Carl Zeiss Meditec) first became commercially available in 2002-2003, the technology has become more proven and widely accepted, and optometrists have realized the full potential of OCT, including both its retina and glaucoma applications.
Spectral domain OCT (SD-OCT) is the newest imaging technology. This technology is also known as Fourier domain, which is named after Baron Jean Baptiste Joseph Fourier, a French mathematician who developed the specific analytic process. Along with the expansion in technology, there has been an increase in the number of instruments available.
The Stratus OCT is the only commercially available TD-OCT. Yet, there are numerous SD-OCT devices commercially available, including the Cirrus HD-OCT (Carl Zeiss Meditec), 3D OCT-1000 (Topcon), Spectralis OCT (Heidelberg Engineering), the RtVue (OptoVue) and the Bioptogen system (Bioptogen Inc.). All are based on the same principles but with subtle differences.
Spectral domain OCT displays greater detail of retinal layers. Through the use of a broader light source, SD-OCT may yield up to a 3m axial resolution image.
Courtesy: Carl Zeiss Meditec
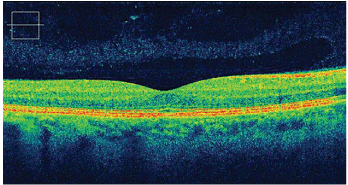
Inherent Advantages
Spectral domain technology allows for unprecedented image capture speed and resolution, along with improved analytic capability. These inherent advantages play an important role in the further evolution of OCT:3
Higher acquisition speed. In TD-OCT, the reference arm moves, limiting the speed of the scan. Traditional OCT captures images at a rate of approximately 500
A-scans/second.
By contrast, a stationary mirror allows for the higher acquisition speed of SD-OCT. As the light from the instrument enters the eye, it is reflected back, partly by a reference mirror and partly by the eye itself. The light reflected back is recorded by a spectrometer and converted to produce an image. This allows for 50- to 100-fold less time needed to capture the image.
This increased speed helps eliminate any motion artifacts that may otherwise degrade image quality. Another advantage: Because of faster image acquisition using SD-OCT, the exposure/A-scan required to obtain images is 100 times lower than that of traditional TD-OCT.4
Higher resolution of images. Higher resolution of images is important for evaluating pathologic changes. Resolution is partly enhanced by the improved speed. Both transverse and axial resolution are used to determine the overall resolution of an instrument. Transverse resolution is limited by the optics of the eye, not the technology. Using the technology, axial resolution can be sharpened to as low as 0.75m, depending on the light emitted by the instrument used.5 SD-OCTs simultaneous axial analysisthe gathering of information from both the reference mirror and the tissue being evaluated, rather than sequential analysis results in faster acquisition speed.6
Studies have shown that SD-OCT is better for detecting abnormalities in numerous conditions, from changes associated with uveitis to vitreo-macular traction with epiretinal membrane.7,8
SD-OCTs superior resolution enables the user to identify the photorecepter inner or outer segment junctions and the outer limiting membrane.4 In a recent study, however, TD-OCT identified thicker retinal layerssuch as the retinal nerve fiber layer, inner and outer plexiform layers, and inner and outer nuclear layersjust as well as SD-OCT.9
Higher resolution also results from the denser sampling ability of SD-OCT. Time-domain OCT uses radial scans and performs some log calculations to plot the area in between the actual lines of scanning. This may not provide ample information to detect subtle abnormalities or changes over time, especially after treatment has been administered. In fact, subtle changes may be detected during a single day, and it is important to know whether these are actual changes or just induced through interpolation.10
Also, the higher resolution for SD-OCT make this a useful technology to manage and follow glaucoma. Currently, Cirrus and OptiVue have normative databases available. Cirrus combines the features of GDx as well as traditional retinal nerve fiber layer analysis in a single scan/printout. OptiVue can even segment the ganglion cell later, and has an analysis software availble for this function.
Image registration. SD-OCT offers improved registration vs. TD-OCT. Image registration refers to the ability to truly detect whether subsequent images are of the exact same anatomical areas as previously imaged.
This is addressed with a number of different methods, depending on the exact machine being used. There is also line-by-line registration. Some instruments allow for the scan to be viewed simultaneously with video. Other devices can register line-by-line with photos, and yet others can be simultaneously viewed by placing the photo over the scan.
More information. The higher acquisition speed and resolution of SD-OCT provide more information to interpret than TD-OCT. This allows for some additional software applications. One such option: the ability to strip away different layers of the retina. An example of this is the ability to remove an epiretinal membrane to see the effect on the tissue below, which could potentially explain visual decline. You can also measure retinal thickness utilizing 3-D topographical and volumetric maps.
Time domain OCT of this patients macula (top) appeared normal, showing nothing more than subtle drusenoid changes at the level of the retinal pigment epithelium. Spectral domain OCT (bottom) of the same patient, however, showed irregularities within the RPE layer due to drusen.
Courtesy: Mark Dunbar, O.D.
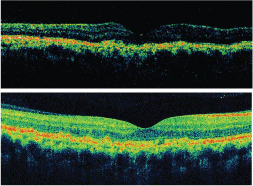
Noteworthy Instruments
Several SD-OCT instruments are available todayeach with subtle benefits and disadvantages. The
A-scan speed ranges from approximately 20,000 to 40,000 scans per second, and axial resolution ranges from 5m to 7m (as reported by the manufacturers) vs. 10m.
Some have added features, such as FA, auto-
fluorescence or indocyanine green angiography capability. They simulate the staining, although the Spectralis can also do actual FA.
Another option: posterior and anterior segment imaging capability. This ability has many potential uses, such as grading anterior chamber angle and fine resolution examination of corneal structures. Anterior segment imaging may even help us evaluate pre- and post-op corneas in refractive surgery patients.
The SD-OCT instruments range in price from approximately $50,000 to more than $100,000. So, one question often comes to mind: Does the average practice truly need this upgrade?
Research shows that both time domain and spectral domain OCT are reliable for macular thickness measurements.11 Also realize that advances in software for the Stratus make it even more useful in the management of glaucoma by improving the ability to document progression and analyzing data, especially from visit to visit. On SD-OCT, however, we may see subtleties that we would have otherwise missed, and may better monitor treatment outcomes.
With more frequent use of any OCT technology, its value will be easier to recognize. We will uncover pathologies that we would have been unable to identify without it. To put the debate of SD-OCT vs. TD-OCT into perspective, we should realize that not long ago, the issue of micron resolution was not even an issue. Why? It was not possible beyond the abilities of a traditional B-scan, which has a resolution of somewhere around 200m.
The future is likely to hold even more advances in OCT technology. Not only is it likely that future instruments will offer improved resolution, but also they will likely be even smaller, as evidenced by the development of the Bioptigen handheld unit. OCT may have further applications, such as the ability to give information regarding tissue oxygenation, be coupled more directly with photography and ultrasound, or be used in conjunction with dyes to further enhance contrast.
As we become more involved in retina comanagement, the need for OCT technology will only increase. Regardless of what is to come, OCTand especially spectral domainwill continue to play a prominent role in the care of our patients.
Dr. Gerson is in private practice in
1. Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res 2008 Jan;27(1):45-88.
2. Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995 Feb;102(2):217-29.
3. Chen TC, Cense B, Pierce MC, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol 2005 Dec;123(12):1715-20.
4. Wojtkowski M, Bajraszewski T, Gorczynska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol 2004 Sep;138(3):412-9.
5. Povazay B, Bizheva K, Unterhuber A, et al. Submicrometer axial resolution optical coherence tomography. Opt Lett 2002 Oct 15;27(20):1800-2.
6. Leder H, Cousins S. OCT Imaging: Advances over the past 5 years and beyond. Retinal Physician 2008 Jan;40-43.
7. Gupta V, Gupta P, Singh R, et al. Spectral-domain cirrus high-definition optical coherence tomography is better than time-domain stratus optical coherence tomography for evaluation of macular pathologic features in uveitis. Am J Ophthalmol 2008 Jun;145(6):1018-22.
8. Koizumi H, Spaide RF, Fisher YL, et al. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol 2008 Mar;145(3):509-17.
9. Ko TH, Fujimoto JG, Schuman JS, et al. Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology 2005 Nov;112(11):1922.e1-15.
10. Frank RN, Schulz L, Abe K, Iezzi R. Temporal variation in diabetic macular edema measured by optical coherence tomography. Ophthalmology 2004 Feb;111(2):211-7.
11. Leung CK, Cheung CY, Weinreb RN, et al. Comparison of macular thickness measurements between time domain and spectral domain ocular coherence tomography. Invest Ophthalmol Vis Sci 2008 Apr 30 [Epub ahead of print].

