Cataracts and ocular toxicity are linked with cancer treatments, according to two separate studies published in the journal Cancer.1,2
The first study revealed that patients who received a hematopoietic stem cell transplantation (HSCT) for childhood cancer have a higher risk of developing cataracts. (HSCT is the collection of ones own [autologous] or a donors [allogeneic] immature blood cells manufactured in the bone marrow that age into red blood cells, white blood cells and platelets.)
A total of 235 cancer survivors (median age: 21) who were treated with HSCT during childhood or adolescenceone-quarter underwent autologous HSCT, and three-quarters underwent allogeneic HSCTwere compared with 705 siblings of childhood cancer survivors. (The most common cancers among these survivors were acute lymphoblastic leukemia and acute myeloid leukemia.) Half the cancer survivors had transplants when they were younger than 10. The median extent of follow-up was 11 years.
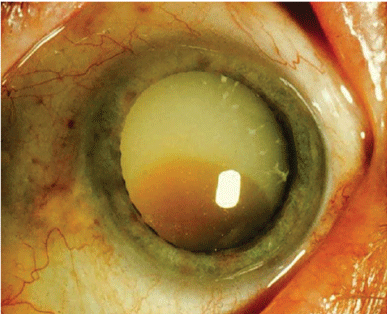 |
|
Childhood cancer patients who were treated with a hematopoietic stem cell transplantation (HSCT) are at greater risk for developing cataracts. |
A total of 2.6% of survivors sustained loss of hearing in one or both ears vs. less than 1% of siblings.
Constant dizziness was cited by 3.4% of the cancer survivors vs. less than 1% of the siblings.
Constant pain was cited by 21% of the cancer survivors vs. 10% of the siblings.
Cancer survivors were 4.3 times more likely to cite coordination problems than the siblings group, 7.7 times more likely to cite swallowing and chewing difficulties and 3.5 times more likely to cite muscle weakness than the sibling group.
The take-home message: If you have a patient who had pediatric cancer and underwent irradiation, monitor him or her closely for cataracts, say the researchers.
The second study revealed that breast cancer patients tend to experience ocular toxicity as the result of chemotherapy-induced ocular irritation.
In this study, researchers culled data on the occurrence and timing of ocular toxicity from the International Breast Cancer Study Group database of 4,948 patients. The patients were randomized to consume the breast cancer drugs tamoxifen or toremifene in combination or alone with either concurrent or sequential chemotherapy and/or hormonal therapy between 1978 and 1999.
More data were collected from patients who developed ocular toxicity after they underwent chemotherapy, but while they were taking tamoxifen or toremifene.
Of the 4,948 patients, 538 reported ocular toxicity during ancillary treatment, primarily during chemotherapy, and 45 patients reported ocular toxicity during hormone therapy alone. Another 30 patients reported ocular toxicity either with no chemotherapy or three months post-chemotherapy, possibly implicating tamoxifen or toremifene.
Other reported findings: retinal alterations without the typical characteristics of tamoxifen toxicity (three patients), cataract (four patients, two with bilateral cataract), impaired visual acuity (12 patients), ocular irritation (10 patients) and optical neuritis (one patient).
Breast cancer patients should be educated on these ocular side effects so they can seek an eye care practitioner who can effectively evaluate their complaints, the researchers conclude.
1. Gurney JG, Ness KK, Rosenthal J, et al. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: results from the bone marrow transplant survivor study. Cancer 2006 Mar 15;106(6):1402-8.
2. Gianni L, Panzini I, Li S, et al.; International Breast Cancer Study Group (IBCSG). Toxicity during adjuvant chemoendocrine therapy for early breast cancer: results from International Breast Cancer Study Group trials. Cancer 2006 Feb 1;106(3):505-13.

