A thorough anterior segment evaluation remains a necessary component of every ocular health exam, and sometimes it can uncover a systemic diagnosis. Cataracts, floppy eyelid syndrome (FES), uveitis, conjunctivitis and episcleritis are just a few presentations that may have an underlying systemic component. In an effort to reduce ocular and systemic morbidity, clinicians must understand the relationship between these ocular findings and any associated conditions so they can clearly communicate with other pertinent care providers.
These systemic conditions are just a few that can present with anterior segment manifestations.
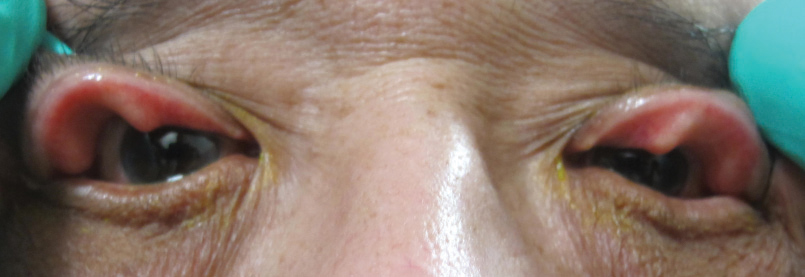 |
| Fig. 1. These upper lids demonstrate significant laxity and easy eversion consistent with floppy eyelid syndrome. |
Wilson’s Disease
This is a rare autosomal recessive disorder—a result of defective excretion of copper by the biliary system—that can lead to copper accumulation in various organs, particularly in the liver and brain.1-4 Excess copper can be toxic and can result in acute liver failure.4 Neurologic manifestations of Wilson’s disease (WD) include personality and cognitive changes and motor dysfunctions such as dystonias, tremors and ataxias.1
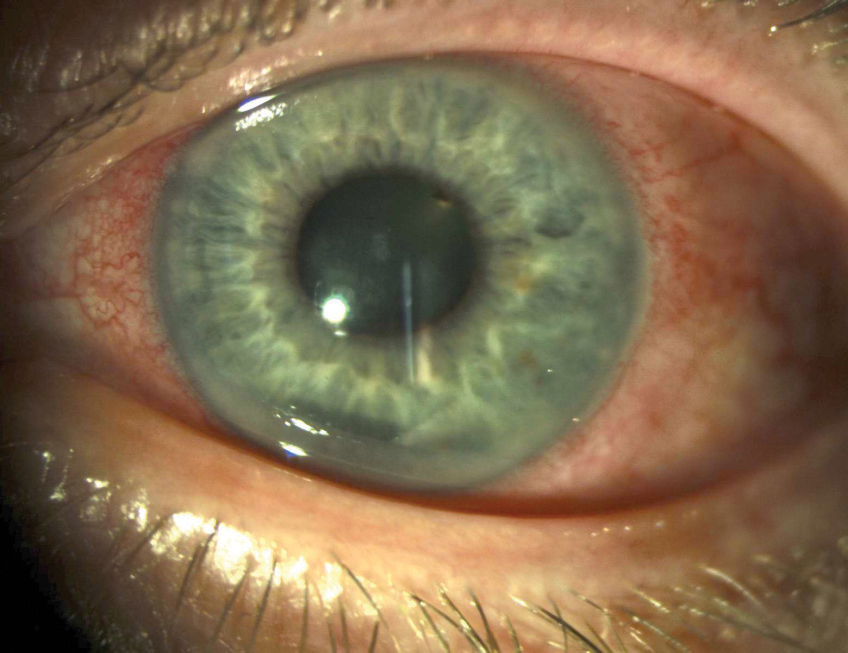
Kayser-Fleischer (KF) rings are the most common ocular manifestation of WD; however, they can occur in other chronic cholestatic disorders as well.4 KF rings, which do not affect vision, appear in the peripheral cornea and are the result of copper deposition in Descemet’s membrane.4-6 The color of the ring can vary from golden, green, ruby or brown and usually forms superiorly and inferiorly before becoming circumferential.4 Only about half of patients with WD isolated to liver involvement have KF rings, though they are present in nearly all patients with neurologic involvement.1
“Sunflower” cataracts, which are not usually visually significant, may also form from copper accumulation in the lens.4-6 Those with WD may also have thinner central corneal thicknesses, steeper keratometric values and shallower anterior chamber depths than normal patients.7
Systemic treatment for WD, generally oral chelating agents, will lead to the resolution of the KF ring.4
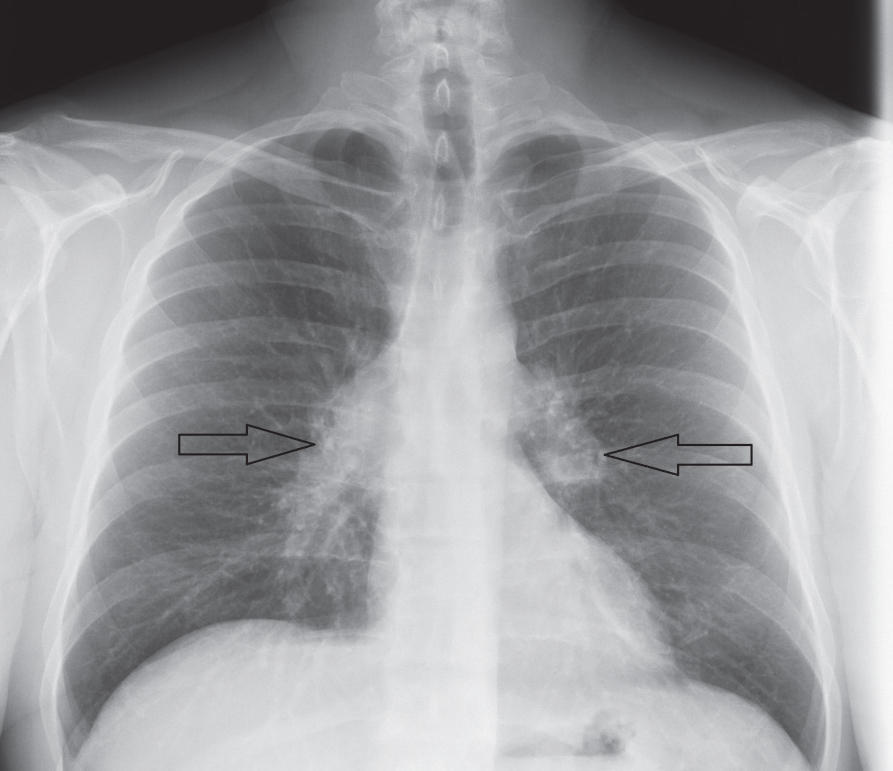 |
| Fig. 2. This patient’s chest x-ray reveals sarcoid-associated bilateral hilar lymphadenopathy (arrows). |
OSA
Affecting approximately 15% of the US population, obstructive sleep apnea (OSA) has become an increasingly common sleep-related breathing disorder.8 The collapse of the pharyngeal airway during the sleep cycle leads to decreased levels of blood oxygenation and increased levels of carbon dioxide. Consequently, frequent arousal from sleep is necessary to allow for normalized blood-gas exchange.9,10 Risk factors for OSA include increasing age, male sex, obesity, craniofacial and upper airway abnormalities and smoking.8,11,12
Floppy eyelid syndrome is a common, yet often underdiagnosed, ocular condition with a well-known association with OSA. Of those diagnosed with FES, 85% have OSA, whereas between 4% and 16% of patients with OSA will present with FES.13,14 Due to increased laxity and easily everted lids, patients with FES often present with complaints of foreign body sensation, burning, tearing and matting, which are commonly worse upon waking as a result of exposure, mechanical irritation or both (Figure 1).15-18 Consequently, corneal and conjunctival complications are common and may include papillary conjunctivitis, superficial punctate keratitis, keratoconus, filamentary keratitis and infectious keratitis.15,17-19
When patients present with suspicious findings, clinicians should obtain an appropriate history from both patient and bed partner, if available, regarding the patient’s sleep habits, particularly in patients without an OSA diagnosis. Snoring, episodes of apnea witnessed by the bed partner, daytime sleepiness, restless sleep, fatigue, nocturia and cognitive deficits are common among those with undiagnosed OSA.17,20,21 Most importantly, an OSA diagnosis carries significant systemic implications such as increased incidence of systemic hypertension, cardiac arrhythmias, coronary artery disease, cognitive dysfunction, congestive heart failure, Type 2 diabetes and increased mortality.15,20,21 Given the systemic risks and the high incidence of OSA in patients with FES, providers should refer patients with FES who have not been diagnosed with OSA for a diagnostic sleep study, especially when symptoms of OSA are present.17,20,22
 |
| Fig. 3. Granulomatous mutton-fat keratic precipitates typical of sarcoid-associated anterior uveitis. Photo: Jennifer Harthan, OD |
Sarcoidosis
This disorder is characterized by the presence of noncaseating granulomatous inflammation, often involving multiple organs.23,24 While upwards of 95% of cases exhibit pulmonary involvement as the most common complication, 30% of patients experience extrapulmonary complications that may involve the eye, skin, liver, nervous system and heart, among others.24-26 Sarcoidosis presents between the ages of 20 to 40 in approximately 70% of those affected, with African Americans having a higher incidence (35.5 in 100,000) compared with Caucasians (10.9 in 100,000) in the United States.27,28
Diagnosis typically requires radiologic investigation of the chest—which reveals bilateral hilar lymphadenopathy and reticular opacities—exclusion of other causal disease and histopathologic confirmation of noncaseating granulomas from a suspected organ site (Figure 2).23,29 Interestingly, the lacrimal gland is a common, easily accessible biopsy site used for diagnosis of sarcoidosis.30
While statistics vary, ocular sequelae occur in 25% to 50% of patients with sarcoidosis and are the initial presenting complication in 5% of all cases.28,31,32 Anterior uveitis, the most commonly detected ocular condition, presents in 66% to 70% of those with ocular involvement.33 Sarcoid-associated anterior uveitis may be monocular or binocular and typically reveals the presence of mutton-fat keratic precipitates, which are characteristic of granulomatous disease (Figure 3).26,31,34 Additional findings that may indicate a more chronic course of sarcoid-associated granulomatous uveitis include Busacca and Koeppe iris nodules (found on the surface of the iris and near the pupillary border, respectively), band keratopathy, posterior synechiae and secondary glaucoma.26,31,33,35 Because 20% of those with uveitis will develop vision loss, prompt recognition and treatment is crucial to the management of this disease.28
While certainly less common than uveitis, other anterior segment findings can present in sarcoidosis. Infiltrative lacrimal gland enlargement often results in keratoconjunctivitis sicca (KCS) and occurs in 15% to 28% of cases.28,31 Conjunctivitis, conjunctival granulomas, scleral nodules, interstitial keratitis, episcleritis and scleritis are also possible.26,31,33
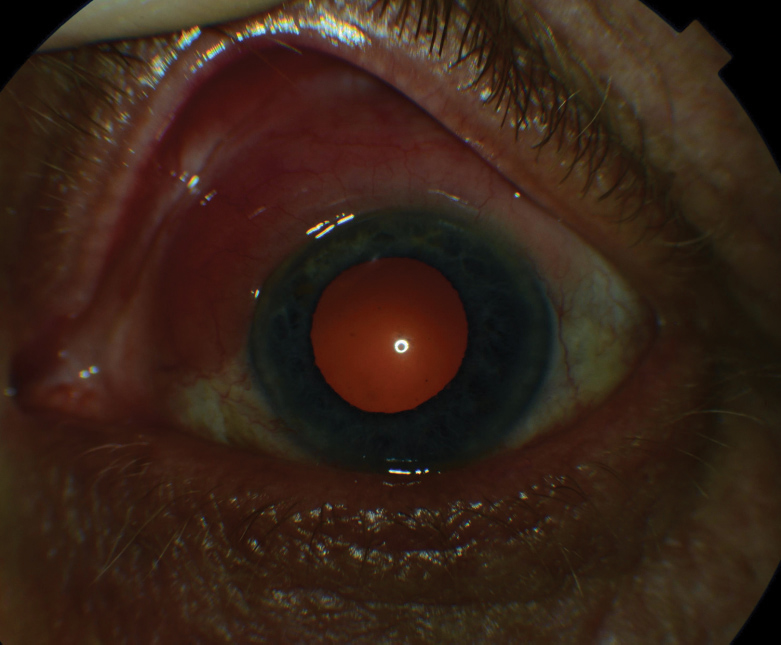
 |
| Fig. 4. This eye demonstrates diffuse conjunctival injection and ciliary flush, commonly seen in HLA-B27-associated acute anterior uveitis. |
HLA-B27-associated Spondyloarthropathies
The spondyloarthropathies are a group of inflammatory disorders that share common clinical characteristics such as inflammation of the axial joints, peripheral arthritis, dactylitis, enthesitis and a negative rheumatoid factor.36,37 The spondyloarthropathies demonstrate a strong association with the human leukocyte antigen (HLA)-B27, a glycoprotein that assists in determining immune response and is associated with an increased incidence of ocular complications, often involving the anterior segment.38,39 Common forms of spondyloarthropathies include ankylosing spondylitis (AS), reactive arthritis, psoriatic arthritis and idiopathic inflammatory bowel disease. Overall prevalence is approximately 0.5% to 1.9% of the population.38,39
Ankylosing spondylitis is the most common spondyloarthropathy, with prevalence approaching 1.4% of the American population.36-38 Nearly 95% of AS patients have a positive HLA-B27 association, whereas other forms of spondyloarthropathies exhibit a range of only 20% to 70%.36,40 Subsequently, ocular complications are common, particularly acute anterior uveitis, which develops in 40% of those with AS (Figure 4).38,40,41 Acute anterior uveitis typically presents unilaterally, with recurrent episodes often involving the same eye; however, future involvement of the fellow eye in a similar, recurrent pattern is not uncommon.42 Unsurprisingly, acute anterior uveitis also exhibits a strong association with HLA-B27.39,42 In cases of acute anterior uveitis, 50% are HLA-B27 positive with incidence increasing to around 70% after recurrent episodes.39,43 Following a literature review of 29,877 patients with any form of spondyloarthropathy, uveitis presented in 32.7% of cases, with acute anterior uveitis being the most commonly detected type of uveitis, further demonstrating the important relationship between this finding and a spondyloarthropathy diagnosis.44
Idiopathic inflammatory bowel disease encompasses both Crohn’s disease and ulcerative colitis. Episcleritis, anterior scleritis, corneal infiltrates and orbital and eyelid edema are anterior segment findings found among those with Crohn’s disease, whereas ulcerative colitis typically involves episodes of uveitis.39,45,46
Reactive arthritis occurs in response to infection and may show ocular involvement as its initial manifestation.38 Arthritis, conjunctivitis and urethritis constitute the classic triad of this disease, although it only occurs in approximately 30% of patients with reactive arthritis.38,39,47 Uveitis, keratitis and episcleritis are also possible findings.48
Psoriatic arthritis, a condition characterized by arthritis and psoriatic skin lesions, develops ocular findings in more than 30% of cases.49 While conjunctivitis is the most common ocular complication, uveitis, dry eye and episcleritis may also present.49,50
 |
| Fig. 5. This left eye demonstrates a large, elevated salmon-colored conjunctival lymphoma involving a large area of the bulbar conjunctiva and limbus. |
Rheumatoid Arthritis
This chronic autoimmune multisystem collagen vascular disease exhibits prevalence approaching 1% worldwide.51 Rheumatoid arthritis (RA) affects women nearly three times more often than men, with peak onset typically occurring between the ages of 40 and 75.52,53
Involvement is typically articular; however, extra-articular complications are also possible and occur in approximately 40% of patients diagnosed with RA.54 Symptoms of early articular RA include a gradual onset of symmetric pain, morning stiffness and swelling of the joints, particularly those of the hands, wrists and toes.55-57
As no specific test confirms a diagnosis of RA, adult patients who exhibit these symptoms for more than six weeks’ duration are highly suspect for RA, particularly in the presence of a positive laboratory test for rheumatoid factor (RF) or anti-citrullinated protein antibodies (ACPA).57 Extra-articular involvement can affect multiple organs including the skin, heart, nervous system, kidneys, liver, lungs and eyes.58,59 Risk factors include increased age, smoking, early disability and the presence of a positive RF and antinuclear antibody (ANA) laboratory findings.54
KCS is the most common ocular complication of RA and occurs in nearly 25% of those with RA.53,59,60 It typically occurs as a result of lymphocytic infiltration of the lacrimal gland due to RA-associated Sjögren’s syndrome.53,61,62 Punctate epithelial keratopathy, mucin stranding and filamentary keratitis are common corneal findings, which, along with conjunctival tissue, progressively exhibit positive staining over the course of the disease.53,62
Episcleritis occurs in less than 1% of patients with RA; however, a more common and concerning ocular complication detected in more advanced cases of RA is scleritis.63,64 Incidence approaches 6.3% in patients with RA, and one study reported 33% of those presenting with active scleritis had a positive diagnosis of RA.63 Corneal complications, which are often associated with scleritis and are potentially sight-threatening, include sclerosing keratitis, limbal guttering, peripheral ulcerative keratitis, acute stromal keratitis and keratolysis.53 Additionally, scleritis-associated anterior uveitis may present.58,62,65
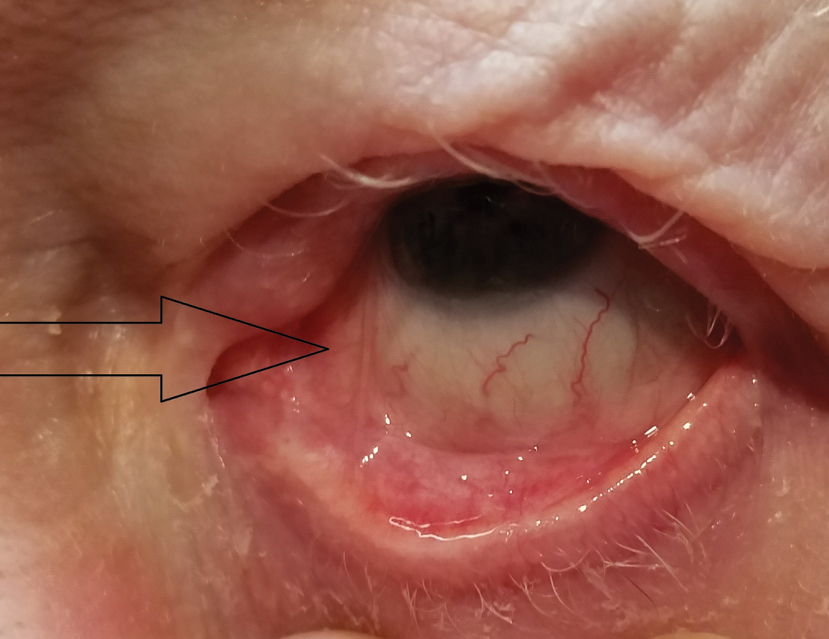 |
| Fig. 6. Focal symblepharon extending from medial palpebral conjunctiva of lower lid to nasal area of bulbar conjunctiva, commonly seen with OCP (arrow). |
Lymphoma
This condition is cancer of the lymphatic system, which begins with lymphocytes (white blood cells). Two main types of lymphoma exist: Hodgkin and non-Hodgkin lymphoma (NHL).66 Hodgkin lymphoma comprises about 14% of malignant lymphomas and is characterized by multi-nucleated giant cells known as Reed-Sternberg cells, but rarely involves the ocular adnexa or anterior segment.67,68 NHL is the most common hematologic malignancy.67 It may be B-cell, T-cell or, rarely, natural killer cell, and several of each subtype exist.67,69
About 1% of all lymphomas and 2% to 8% of extranodal lymphomas develop in the eye’s adnexa—approximately 37% of those are orbital, 25% to 29% conjunctival, 29% lacrimal and 14% involve the eyelid, although lymphoma can also present at other sites within the globe as well.68,70 Special lymphoid tissue within the conjunctiva and lacrimal gland are part of the mucosa-associated lymphoid tissue (MALT) system, from which B-cell NHL can arise.68,71 The risk of developing lymphoma increases in the presence of chronic inflammatory diseases such as Sjögren syndrome, which has a nearly 14-fold increased incidence of NHL.72,73
Lacrimal gland lymphoma may present with eyelid swelling, pain or proptosis and generally appears as a solid enlarged mass.74 Eyelid lymphoma can involve the skin, subcutaneous connective tissue, as well as the orbicularis oculi.69 Lymphoma may also appear as a chronic anterior uveitis.
Conjunctival lymphomas typically present as raised, salmon-colored lesions.71 They may be round or oval when involving the bulbar conjunctiva (Figure 5). In the fornix, they tend to conform to the forniceal contour, with the inferior fornix being the most commonly involved conjunctival site.75,76 While they are usually smooth, the lesions can appear lobulated or present similar to a follicular conjunctivitis.66 They are bilateral in 20% to 38% of cases, most often occur in the 5th to 7th decades of life and affect females slightly more often than males.66,70
Most conjunctival lymphomas are NHL B-cell with one of four major subtypes (in decreasing order of frequency): extranodal marginal zone lymphoma of the MALT system, follicular, mantle cell and diffuse large B-cell lymphoma.70 Extranodal marginal zone and follicular lymphomas are low-grade, whereas mantle cell and diffuse large B-cell are high-grade malignancies.66,70,77 Tissue biopsy of the suspected site is necessary for diagnosis and subtype differentiation, which will also help to guide treatment.71
Most conjunctival lymphomas are primary, with approximately one-third of those having regional lymph node and/or systemic involvement.66 Additionally, 20% of those with local conjunctival lymphoma will develop systemic lymphoma; therefore, a referral for appropriate systemic investigation is critical.66,78
Lyme Disease: An OD’s First-hand AccountBy Cheryl G. Murphy, OD, Contributing Editor What started as a dull ache behind my right eye soon turned into the worst headache of my life. I became feverish, fatigued and weak. I saw “wiggling worms of light” dancing in my inferior visual field. My condition continued to decline—I lost all appetite and could barely get out of bed. I thought maybe I had the flu, but it was June and flu season had passed. On the fourth day I went to the emergency room (ER) and was diagnosed with viral meningitis. The ER doctor explained that any virus at any time (even viruses that cause the common cold or a cough) can sporadically decide to invade the brain and spinal cord. I asked how he knew it wasn’t bacterial meningitis. He said I “would have been dead by the fourth day.” After extensive testing, scans and blood work, all of which came back negative, I was discharged and instructed to wait the virus out. Stay hydrated and rest, the doctor said. Once home, I decided to take a cool shower. It was then I noticed a red rash on my right hip. It wasn’t a bulls-eye, but I immediately thought of Lyme disease. We had just moved into a new house that sat on an acre of woods, and deer were known to forage for food throughout the neighborhood. Although my blood test for Lyme had come out negative, I visited my general physician and pleaded with her to start me on the recommended course of doxycycline. She agreed, but only to a three-day dose; she would repeat blood work for Lyme and increase to the full course of antibiotics only if it came back positive. The new blood work came back positive. Luckily, I caught it relatively early and after completing a 21-day course of doxycycline, have not suffered any long-term effects. With the number of ticks carrying Lyme disease on the rise in the Northeast, it is essential for us as optometrists to be aware of the possibility that a seemingly harmless or vague symptom such as dull eye pain could be the beginning of something much more serious. |
Mucous Membrane Pemphigoid
This is a multisystem autoimmune subepidermal blistering disorder that affects the mucous membranes at the ocular, oral, nasopharyngeal, tracheal, esophageal, anogenital and genitourinary orifices.79 Common findings include inflammation, subepithelial blistering and cicatricial shrinking of the affected tissue.79 The incidence of mucous membrane pemphigoid (MMP) is low, at 1.16 to 2 per million.80 Conjunctival involvement is referred to as ocular cicatrical pemphigoid (OCP) and is detected in approximately 70% of patients with MMP.79-82
OCP causes deposition of immunoglobulin and complement in conjunctival basement membrane.81,83 Circulating autoantibodies bind to antigens within the basement membrane and activate the complement cascade. Cellular response then leads to subepithelial bullae formation and scarring.83 As the condition progresses, the eye develops symblepharon with shortening of the fornices; this leads to xerosis, tear abnormalities, meibomian gland dysfunction and abnormalities of the eyelid margin, which also can cause mechanical trauma to the surface of the eye (Figure 6).83 Endstage corneal damage can occur, resulting in decreased vision from vascularization and opacification.81,84
Early diagnosis can be challenging, as the initial presentation is usually a unilateral, chronic, recurrent, papillary conjunctivitis; as a non-specific finding, this contributes to the typical 2.8 years until arriving at a correct diagnosis of OCP.83,85 By the time of diagnosis, most patients will already have significant bilateral forniceal shortening and ocular morbidity.85 A high level of suspicion for OCP should be present in any unexplained, chronic, recurrent conjunctivitis, especially in the presence of subepithelial scarring of the conjunctiva. Conjunctival biopsy with pathologic analysis can help to confirm a diagnosis of OCP, although false negatives are possible.81,83
Treatment is difficult and is applied in a step-wise fashion to minimize side effects.84 Topical treatment alone is rarely successful for long-term control; oral immunomodulatory or biologic therapies are typically indicated, and management should be provided by a specialist familiar with the administration and monitoring of these therapies.84
Detection of anterior segment manifestations of systemic disease is a significant opportunity for ODs to participate in life-changing, and in some cases life-saving, care. Because the related systemic condition may be previously unknown to the patient, it is our responsibility to accurately diagnose, manage and communicate our findings and concerns to the appropriate medical specialty to ensure timely care.
Drs. Fliegel, Weidmayer, Lewis and Seng are staff optometrists in the VISN 10: VA Healthcare System – US Department of Veteran Affairs.
Contents in this publication do not represent the views of the US Department of Veterans Affairs or the United States government.
1. Biswas S, Paul N, Das SK. Nonmotor manifestations of Wilson’s disease. Int Rev Neurobiol. 2017;134:1443-59. |

