When optometrists think of the ciliary body, it is likely that they will first think of accommodation or glaucoma. In the last several years, however, three recent studies reported that the ciliary body is not the same for all of our patients. In fact, all three of these studies have shown that the ciliary body tends to be thicker in patients with myopia.
In 2005, Cristiano Oliveira, M.D., Department of Ophthalmology, The New York Eye and Ear Infirmary, and coworkers showed that the ciliary body was thicker in myopic patients than in emmetropic or hyperopic patients.1 In 2008, our laboratory in Columbus, Ohio, published a paper that showed that the ciliary body was thicker in children with myopia.2 Finally, in early 2009, Orkun Muftuoglu, M.D., Department of Ophthalmology, Ankara University School of Medicine, Turkey, and associates published a study that reported the more myopic eye had a thicker ciliary body in patients with unilateral high myopia.3
As we explore this topic further, we need to consider several fundamental questions:
1. Why didn’t we notice that there were differences in ciliary body thickness before?
A study participant
views an external target while the anterior segment optical coherence
tomography (AS-OCT) instrument is scanning the nasal sclera.
As an optometrist, you might have expected the opposite relationship between refractive error and ciliary body thickness, because other ocular structures, such as the choroid, are thin in myopia.4 Instead, the ciliary body seems to be the only structure within the eye that thickens with myopia development.
Another curiosity of this line of research is that until 2005, no one knew that the ciliary body thickness varied with refractive error status.
Because the ciliary body is tucked in behind the iris, it is not visible during a dilated fundus examination. Traditionally, it was only visible upon imaging with an ultrasound biomicroscope.
While ultrasound biomicroscopy has been used to study the relationship between refractive error and ciliary body thickness in recent adult studies, ultrasound biomicroscopy has not been feasible in large studies of children.1,3
Ciliary body thickness was not measured in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study, which was a large, multi-ethnic study of myopia’s etiology that was funded by the National Eye Institute. No one knew ciliary body thickness might be important at the time the study was designed, and it would not have been feasible to image it regardless because ultrasound biomicroscopy is too uncomfortable for children and anterior segment optical coherence tomography (AS-OCT) did not exist.
Fortunately, we have been able to visualize the ciliary body in children because of recent advances in imaging technology. These advances have allowed us to view the anterior segment of the globe through AS-OCT.2,5
2. How is it possible to image the ciliary body in children?
If you have read any articles on anterior segment OCT, you probably are aware that the ciliary body was not visible in these images because OCT imaging cannot penetrate through pigmented structures. While this is true, our laboratory has been able to successfully view the ciliary body through the sclera.
The children do not look at the target inside the instrument; instead, they look at a temporal target located outside of the machine. We can then image the ciliary body through the nasal sclera. This avoids iris pigment and provides images that can be used to further study the
relationship between refractive error, accommodation and ciliary body thickness in children. We have also used AS-OCT (specifically, Visante OCT [Carl Zeiss Meditec]) in studies of myopia and presbyopia in adults because it is non-contact and much more comfortable for the patient than ultrasound biomicroscopy.
3. Does ciliary body thickness matter?

Dr. Bailey’s laboratory in Columbus, Ohio, has successfully been able to view the ciliary body of children through the sclera.
While several ongoing studies are examining this topic, there are many potential clinical applications of this research.
There are reasons why the thickness of the ciliary body may matter in several aspects of eye care; for example, the ciliary body is probably important in both accommodative lag and hyperopia.
4. Is a thicker ciliary body the source of accommodative lag in myopia?
A well-known association exists between accommodative dysfunction and myopia development in children. Multiple studies have reported that children with myopia have higher accommodative convergence per diopter of accommodation (AC/A) ratios than children without myopia, and higher AC/A ratios appear in these children prior to the onset of myopia.6-9 Additionally, accommodative lag has also been associated with myopia development.7,10
Whether or not these accommodative problems are a cause or an effect of myopia is a hotly debated topic. Still, the anatomical source of accommodative problems in myopia—whether cause or effect—is unknown. So, we are left with a question: What component of the accommodative system is deficient such that children with myopia lag in accommodative effort?
Certainly, we can speculate that the ciliary muscle is a prime candidate for a source of accommodative lag. The ciliary muscle is, of course, the muscle that generates accommodation. We are actively examining elementary school children to determine if AC/A ratio and accommodative lag are related to the size of the ciliary muscle. Our current working hypothesis is that the ciliary muscle becomes hypertrophic as the eye is elongating during myopia progression.
While the true cause of the thicker ciliary muscle is unknown, there are two possibilities that would explain the increase in thickness in myopia: either hypertrophy of hyperplasia. For example, accommodation is impaired while the eye is growing in myopia, but it recovers once the eye stops growing in adulthood.11,12 Because chronic hypertrophy is known to reduce the contractile properties of smooth muscle, a transient loss of accommodation could be explained by the ciliary muscle becoming hypertrophic during childhood and myopia progression, but remodeling and recovering full function later in life.13 The other possibility is that marked hyperplasia might also lead to a reduction in the ability of the muscle to contract with remodeling and a recovery of function later.
Outside of the rare tumor, no childhood diseases of the ciliary muscle are known. That being said, an absence of hypertrophy or hyperplasia potentially would make the ciliary muscle the only smooth muscle in the human body that does not have an associated smooth muscle disease. Smooth muscle hypertrophy and hyperplasia occur in humans as a disease process in the lungs (severe asthma), blood vessels (hypertension), pyloric sphincter (infantile pyloric stenosis), intestines (Hirschsprung’s disease), bladder (overactive bladder) and uterus (pregnancy).13-20 Either the ciliary muscle is a unique smooth muscle, or we have noticed poor function in the ciliary muscle because we could not see it.
5. Does it matter that the ciliary body is thinner in some hyperopic eyes?
Our 2008 publication on ciliary body thickness in children did not have enough hyperopic participants to compare hyperopic and emmetropic children. However, Dr. Oliveira’s study did report that the ciliary body was thinner in adults with hyperopia.1
Does a thinner ciliary body in hyperopia mean those individuals will have less accurate accommodation? Our lab is working to answer this question. In the meantime, because there are potential differences in accommodative function related to a thinner ciliary body should be considered clinically. Certainly, greater levels of accommodative lag have already been associated with higher levels of hyperopia.21 Also, some research suggests that correcting hyperopes with high accommodative lags may improve reading fluency.22
All of this taken together suggests that you should certainly measure accommodative lag in higher hyperopes when deciding if you should prescribe glasses, because hyperopes may have additional disadvantages due to a thinner ciliary muscle.
6. What are the future directions for research?
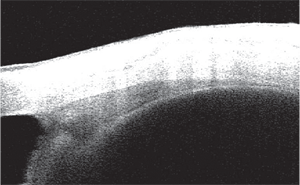
Image of an AS-OCT image of the nasal ciliary body.
For future investigations of the role of the ciliary body in refractive error development and accommodative function, we are testing children in elementary schools annually to monitor changes in refractive error, axial length, accommodative function and ciliary body thickness. Also, we have developed a method for determining how ciliary body thickness changes with accommodation, and we will measure children with hyperopia and myopia to determine if there are changes in ciliary muscle contraction in these eyes.23
Finally, we are evaluating animal models of myopia to determine if they show the same kinds of ciliary body changes that are found in humans, and to determine if we see changes in the ciliary muscle tissue that resemble smooth muscle hypertrophy.
Dr. Bailey is an assistant professor at The Ohio State University College of Optometry, where she teaches courses in practical optometry and contact lenses. She has received funding for her research on the ciliary body and myopia from the Ohio Eye Lions Research Foundation, and she is a KL2 Scholar at the Center for Clinical and Translational Sciences at Ohio State. She has no commercial relationships to disclose.
1. Oliveira C, Tello C, Liebmann JM, Ritch R. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol. 2005;140:324-5.z
2. Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008;49:4353-60.
3. Muftuoglu O, Hosal BM, Zilelioglu G. Ciliary body thickness in unilateral high axial myopia. Eye. 2009;23:1176-81.
4. Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445-50.
5. Schultz KE, Sinnott LT, Mutti DO, Bailey MD. Accommodative Fluctuations, Lens Tension, and Ciliary Body Thickness in Children. Optom Vis Sci. 2009;86:677-84.
6. Gwiazda J, Grice K, Thorn F. Response AC/A ratios are elevated in myopic children. Ophthalmic Physiol Opt. 1999;19:173-9.
7. Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005;82:273-8.
8. Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000;41:2469-78.
9. Mutti DO, Mitchell GL. The response AC/A ratio before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47.
10. Mutti DO, Mitchell GL, Hayes JR, et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837-46.
11. Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol Opt. 1998;18:13-20.
12. Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res. 1995;35:1299-304.
13. Hillemeier C, Biancani P. Mechanical properties of obstructed colon in a Hirschsprung’s model. Gastroenterology. 1990;99:995-1000.
14. Benayoun L, Druilhe A, Dombret MC, et al. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360-8.
15. Jackson CL, Schwartz SM. Pharmacology of smooth muscle cell replication. Hypertension. 1992;20:713-36.
16. Owens GK, Rabinovitch PS, Schwartz SM. Smooth muscle cell hypertrophy versus hyperplasia in hypertension. Proc Natl Acad Sci USA. 1981;78:7759-63.
17. Oue T, Puri P. Abnormalities of elastin and elastic fibers in infantile hypertrophic pyloric stenosis. Pediatr Surg Int. 1999;15:540-2.
18. Yamamoto A, Kino M, Sasaki T, Kobayashi Y. Ultrasonographic follow-up of the healing process of medically treated hypertrophic pyloric stenosis. Pediatr Radiol. 1998;28:177-8.
19. Galvin DJ, Watson RW, Gillespie JI, et al. Mechanical stretch regulates cell survival in human bladder smooth muscle cells in vitro. Am J Physiol Renal Physiol. 2002;283:F1192-9.
20. Seidel CL, Weisbrodt NW. Hypertrophic response in smooth muscle. Boca Raton, Fla.: CRC Press; 1987.
21. Hayes JR, Mutti DO, Jones LA, et al. Accommodative lag in corrected and uncorrected hyperopia. Optom Vis Sci. 2002;79:s196.
22. Sims J, Kleinstein R. Accommodative lag and reading fluency. Optom Vis Sci. 2007;84:E-Abstract 075019.
23. Lossing LA, Richdale K, Sinnott LT, Bailey MD. Changes in ciliary body thickness with accommodation. Optom Vis Sci. 2009;86:E-Abstract 95815.

