Technology in cataract surgery continues to evolve to meet the needs of both doctors and patients. More than ever, patients expect exceptional outcomes, including less dependence on spectacles. The precision of pairing femtosecond laser-assisted cataract surgery (FLACS) with a multitude of premium intraocular lens (IOL) options, for example, is one way doctors are incorporating new technology to deliver the outcomes their patients expect. Many new options are available that are improving the cataract surgery experience for all involved, and understanding them is key to proper patient education and comanagement.
This article reviews FLACS, premium IOL technology, future IOL designs, postoperative advances and current controversies in cataract surgery to keep you up-to-date on the evolving landscape of cataract surgery.
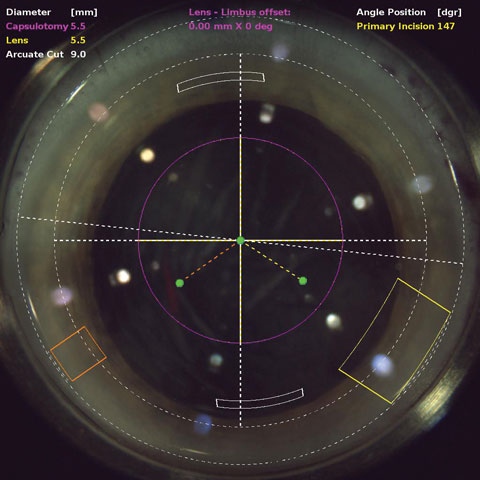 |
| OCT during laser cataract surgery allows the cataract surgeon to plan the location and depth of incisions. Click image to enlarge. |
Laser Cataract Surgery
FLACS is still a developing technology, and while research continues to evaluate its effectiveness, current findings only suggest it is noninferior relative to manual cataract surgery.1 Femtosecond laser technology in contrast to manual methods takes certain parts of the cataract procedure out of the surgeon’s hands, such as corneal incisions, the capsulotomy and softening of the lens. Potential advantages, still being debated, include a precise shape and size of the capsulotomy, custom lens fragmentation patterns, a reduction in endothelial cell loss, customized and precisely placed corneal incisions and improved refractive stability and predictability.1 Currently there are five FLACS platforms on the market: LenSx (Alcon), LensAR (LensAR), Catalys (Abbott), Victus (Bausch + Lomb) and LDV Z8 (Ziemer).2
The capsulotomy is arguably the most important step in the cataract surgery procedure, as the size of the capsulotomy is key to optimizing the position and performance of an IOL. A capsulotomy that is too small has the risk of anterior capsule fibrosis and a hyperopic shift, while a too large capsulotomy can increase rate of tilt, decentration and posterior capsular opacification.3 All of these issues can lead to a less than desirable outcome for patients, and in some cases the need for a lens exchange.4-6 In addition, the capsulotomy plays a crucial role in predicting the effective lens position, which is important in IOL power calculations.7 A difference of only 1mm in lens position can lead to a 1.25D change in refractive error.3,8 With toric and multifocal IOLs, the margin of error is even smaller. A tilted, decentered or rotated IOL can cause a significant deviation from the desired refractive outcome, and can make it difficult to tolerate visual aberrations such as halo and induced coma.9,10
Can FLACS minimize many of these issues with a laser-incised capsulorhexis? One study found no difference in predictable lens position error when comparing traditional phacoemulsification with FLACS, but it did find higher refractive stability and IOL centration.11
A femtosecond laser can also be used to segment the nucleus and place pattern cuts on this structure to soften harder cataracts using less ultrasound energy. These treatments theoretically decrease complications relative to the manual technique, but debate exists. Researchers compared corneal endothelial cell loss after fluid-based vs. ultrasound phacoemulsification and concluded there was significantly lower endothelial cell loss after phaco with a fluid-based vs. ultrasound system.12 Others have recently compared endothelial cell loss rates between phacoemulsification and FLACS and found similar results of no difference between both modalities.13,14 FLACS has been shown to have less day-one edema, which is always something eye care providers watch for.15
Although FLACS has many potential advantages, many studies bring into question the benefit of the technology. A recent study that assessed the visual outcomes of 988 eye that underwent FLACS and 888 eyes that underwent standard phacoemulsification cataract surgery found that, six months postoperatively, there was no clinically meaningful visual benefit to FLACS over standard phacoemulsification.16 Best-corrected visual acuity was slightly better with FLACS than standard phacoemulsification (20/24.5 vs. 20/26.4), and the researchers concluded that, given the refractive outcomes, FLACS is not currently cost-effective.16
The same study also looked at safety issues and found that in the FLACS group there were 15 anterior capsule tears compared with three in the standard phacoemulsification group and 11 posterior capsule tears vs. two in the standard phaco group.16
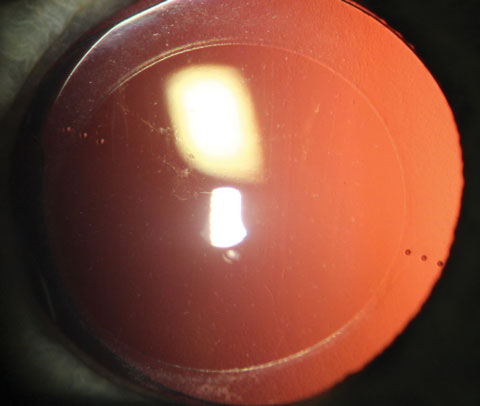 |
| A toric IOL with the axis markings visible. |
Premium IOL Options
Many new options exist, providing better visual outcomes for wide array of patients, including those with astigmatism and presbyopia:
Toric IOLs
Emmetropia can be achieved for patients with myopic or hyperopic refractive errors by selecting the appropriate spherical IOL lens power. However, nearly 20% to 30% of patients who undergo cataract surgery have corneal astigmatism of 1.25D or higher, and nearly 10% of patients have 2.00D or higher.17 Toric IOLs offer patients the opportunity to correct corneal astigmatism at the time of cataract surgery and increase the likelihood of spectacle independence for distance vision. Multiple toric IOL models are available, including the AcrySof IQ Toric (Alcon), Tecnis Toric (Abbott) and Staar Toric IOL (Staar Surgical).
New multifocal toric IOLs take the correction of astigmatism a step further by not only correcting distance vision but also near and intermediate vision. The AcrySof IQ Restor Toric multifocal (Alcon) is an example of this type of technology, although not yet FDA approved in the United States. In one study, patients with 1.00D or higher astigmatism with a multifocal IOL showed a compromise to both distance and near visual acuities.18 Such results highlight the importance of optimal astigmatism correction in patients with low amounts of corneal toricity.
Although minimal, complications are consistent with those found with monofocal IOLs, except alignment is significantly more crucial with a toric IOL. The efficacy of toric IOLs is dependent on the position of the IOL relative to the intended alignment axis. Residual astigmatism is induced for every degree of misalignment from the intended alignment axis. For every degree that the lens is off, the patient loses 3.3% of astigmatism correction. If a toric lens is off by 30 degrees, it has no cylindrical effect and acts similar to a spherical IOL. Misalignment will affect a higher-powered toric IOL more significantly than a lower-powered IOL. For example, if an AcrySof T3 (Alcon) that corrects 1.03D of astigmatism is misaligned by 15 degrees, there will be a loss of 0.51D or roughly 50% of its astigmatism correction. In contrast, if an AcrySof T9 (Alcon) that corrects 4.11D of astigmatism is misaligned by 15 degrees, there will still be a 50% loss of astigmatism correction, but the magnitude will be much higher at 2.05D. A misalignment of more than 10 degrees is generally regarded as an indication for surgical repositioning.19 The two main factors accounting for misalignment are inaccurate alignment of the IOL during surgery and postoperative rotation of the IOL.19
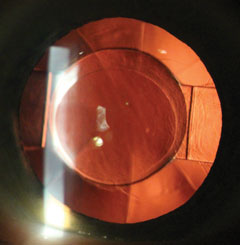 |
| With this Crystalens IOL, note the modified plate haptic with the hinges to allow backward and forward movement. Click image to enlarge. |
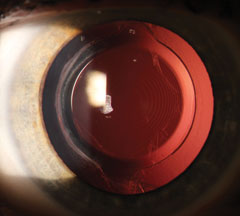 |
| Note the concentric rings common with multifocal IOLs. Click image to enlarge. |
Determining how to correct the residual astigmatism can be challenging. Generally, it can be corrected by rotating the IOL, laser vision correction, IOL exchange or limbal relaxing incisions. However, cross cylinder effects complicate matters, and in these situations it can be helpful to use an online toric IOL calculator (e.g., astigmatismfix.com) to help decide if lens rotation is necessary.
Multifocal IOLs
All patients older than 40 know presbyopia can be a frustrating process, and those who want to ditch their spectacles for both distance and near now have many multifocal IOL options from which to choose. Multifocal IOLs allow multiple focal distances independent of ciliary body function and capsular mechanics by using diffractive optics that split light between distance, intermediate and near. Once placed in the capsular bag, the function of the multifocal lens will not change over time.
Current multifocal IOL technology (e.g., AcrySof Restor IQ, Alcon; Tecnis Multifocal, Abbott) is available in high-add and low-add configurations. Practitioners can provide patients with a large range of vision and decreased dependence on readers by using a low add in one eye paired with a high add in the contralateral eye.
The challenge of multifocality remains with preserving optical quality for patients. An overall healthy eye is required for multifocal IOLs to work best. Macular function and tear film quality must be examined and treated, if necessary, to achieve optimal outcomes with multifocal IOLs. In situations where contrast sensitivity is reduced permanently, as can be the case with patients with moderate to severe glaucoma, multifocal IOLs should be used with caution.
Another major challenge for multifocal technology is reducing or eliminating phenomena such as glare and halos. Patient selection can be challenging in these situations, as it is more of an art than a science. Practitioners should remain cautious with demanding patients, especially those expecting perfect visual performance in low light conditions. Fully counseling patients on the chances of needing part-time spectacle correction for night-time vision or reading in dim illumination is imperative. If the patient is unwilling to accept this as a reality, offering monfocal options may be the best way to proceed.
Clinicians should also consider lifestyle and visual expectations when deciding if a patient is suitable for a multifocal IOL. This involves a detailed history regarding the patient’s work and leisure activities, the amount of time spent doing each and a ranking of how important each is to the patient.
Eye care providers must also understand the functional benefits and limitations of the IOL. Specific multifocal IOLs have certain strengths and weaknesses concerning their ability to function adequately at certain distances, with residual amounts of corneal astigmatism or when there is IOL decentration.20
 |
| A misalignment of 15 degrees with a toric IOL is simulated in this image. With 15 degrees of misalignment, the IOL loses 50% of its astigmatic correcting power. |
Accommodative IOLs
These lenses are intriguing because the technology addresses presbyopia—and astigmatism, in certain IOLs—by attempting to mimic the eye’s natural method of focusing. Clinical studies indicate that restoration of accommodation may be achieved, to some extent, with axial movement of the lens optic within the capsular bag.21,22 The IOL needs to have forward-backward axial movement or flexibility in its shape or thickness to effect change in focal point from distance to near vision. Examples of these types of IOLs include the Crystalens AO (Bausch + Lomb) and the Trulign Toric IOL (Bausch + Lomb), which merges presbyopia and astigmatism correction.
One major advantage of accommodative IOLs is a greatly decreased risk of visual aberrations such as halos or contrast sensitivity loss compared with multifocal IOLs because the brain is not forced to choose between different images, as is the case with multifocal lenses.
Challenges do exist, the main one being achieving more near power. So far, no accommodative device can perfectly duplicate the eye’s accommodative mechanism, so patient education on near point ability with these lenses is imperative. Studies also show accommodative lenses are associated with more posterior capsular opacification, which can induce asymmetric vaulting, leading to lens tilt and ultimately a decrease in visual acuity.22
Extended Depth of Focus (EDOF) Lenses
Another emerging technology, EDOF IOLs (e.g., Tecnis Symfony, Abbott; IC-8, AcuFocus; WIOL-CF, Medicem), use new designs to improve the range of vision without splitting light rays. Much like accommodative and multifocal IOLs, this technology is designed with the presbyopic patient in mind. Instead of the single focal point of accommodative lenses or two distinct foci of multifocal lenses, the EDOF IOL creates one elongated focal point, intended to smooth out the dips in the defocus curve common with other presbyopia-correcting IOLs. Since EDOF IOLs don’t split light, patients experience less glare and halos, but the intensity of near vision does not match that provided by multifocal lenses.
According to one study conducted on the Symfony, 99.6% of patients had binocular distance UCVA of 20/40 or better at distance, 96.6% had binocular intermediate UCVA of 20/25 or better and 95.9% of patients had binocular near UCVA of 20/40 or better.20 In this study, 97% of 31 subjects indicated they would elect to have the lens implanted again.23 While the Symfony IOL is approved for use in the US and includes a toric design—the Tecnis Symfony Toric IOL (Abbott)—the IC-8 and WIOL-CF have yet to receive approval.
Controversies in Cataract SurgeryIn addition to the lack of consensus on the proper role of FLACS—some surgeons perform it routinely, some reserve it only for more challenging cases and a sizable contingent still favor manual techniques—other debates have captured the attention of both ODs and MDs. • Dropless Cataract Surgery. One of the biggest concerns for patients and eye care providers is postoperative eye drops. Compliance issues remain a major concern, and patients’ lack of adherence to their medication regimens can lead to complications. One study found that 50% of patients took less than half, and 20% took less than a quarter, of their prescribed medications after cataract surgery.24 With recent advances in intracameral or intravitreal injections at the end of cataract surgery, there is hope for increased compliance while decreasing the number and cost of postoperative drops and the incidence of complications. Intracameral injections after cataract surgery are well accepted in Europe, with nearly 74% of European ophthalmologists adopting the use.25 The injection consists of a broad-spectrum antibiotic, typically moxifloxacin, and a steroid such as triamcinolone. The medication is injected directly behind the IOL, through the zonules and into the vitreous cavity. Injections minimize the most important risk factor in cataract surgery, endophthalmitis. A large study compared intracameral antibiotics vs. topical antibiotics after cataract surgery and found with the addition of intracameral antibiotics there was a 22-fold decrease in endophthalmitis rates.26 The study concluded at five years, and the participating surgeons’ use of intracameral antibiotics climbed from 11% to 100%.26 A second benefit is cost-effectiveness, as the patient does not have to purchase both a topical steroid and topical antibiotic. Finally, it minimizes the instillation of topical drops, which is particularly helpful for patients with physical limitations that make instilling drops difficult. Common concerns regarding the use of an intraoperative injection include the risk of intraocular pressure (IOP) spikes and the efficacy of controlling surgically-induced inflammation and cystoid macular edema. A study of 1,575 eyes that received an injection of antibiotic and steroid found that the mean IOP was 21.8mm Hg on the day of surgery and 14.5mm Hg at three weeks post-procedure.5 No eyes required ocular hypotensive treatment due to a steroid response, the rate of CME was 2%, inflammation rate was 2.5% and, in those eyes, the use of topical steroids was required to reduce the inflammation.26 • Bilateral Cataract Surgery. Although most surgeons delay surgeries between eyes to avoid potentially blinding a patient with a bilateral complication, there is a growing number of surgeons who believe simultaneous bilateral cataract surgery is better for patients because of rapid patient rehabilitation and the economy of a one-stop approach. Studies show various economic and quality of life arguments that favor bilateral cataract surgery.27 This procedure is not well accepted by health insurers or providers despite these studies, mainly due to safety concerns.28 A large multicenter randomized clinical trial reported a very low rate of intraoperative and postoperative complications, including a low rate of CME.29 The counterarguments revolve around safety. Concerns over endophthalmitis and toxic anterior segment syndrome can’t be ignored. A second counterargument is the ability to consider the refractive outcome. By delaying surgeries, the surgeon can consider the refractive outcome in the first eye and modify the IOL power in the second eye.30 The debate will continue, but it is hard to argue with the compelling evidence of economic and quality of life advantages for patients. |
The Future
The future is bright for IOL technology, particularly for two categories: devices with a modular multicomponent category and those where the optic is adjusted postoperatively with a secondary device. Both of these allow customized prescriptions for the patient at the time of the primary surgery or at a time in the postoperative period.
Multicomponent IOLs have a base unit—that serves as a holding device to secure the optic—which is placed within the capsular bag similar to a standard cataract procedure. The design is intended to allow a safe and easy exchange of the optic component to reduce residual postoperative refractive error at the time of surgery or postoperatively, potentially minimizing the need for secondary touchups and offering adjustability postoperatively.
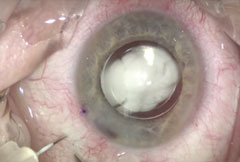 |
| A transzonluar injection of TriMoxi after cataract surgery. Click image to enlarge. |
IOLs that provide adjustments postoperatively with a secondary device have many advantages, such as the decreased risk of infection because the surgeon does not have to re-enter the eye to adjust or exchange the implant. The design also eliminates secondary adjustments using a corneal refractive procedure, leaving the anatomy of the cornea untouched and eliminating the recovery process that comes with those procedures.
The Light Adjustable Lens (Calhoun Vision) is one example of this type of IOL technology, which is currently going through FDA clinical trials in the United States. A patient undergoes traditional cataract surgery and has the monofocal IOL implanted into the capsular bag. The IOL’s silicone macromers, which are distributed evenly throughout the lens, are photosensitive to near-ultraviolet wavelength of energy. If the patient has residual refractive error in the postoperative period, a secondary device can apply the near-ultraviolet wavelength of energy to the IOL to change the distribution of the macromers and correct the refractive power. A myopic adjustment occurs when the pattern puts more energy in the periphery of the lens, and vice versa for a hyperopic adjustment. Cylindrical adjustments are also possible. The surgeon can adjust the IOL multiple times until the lens power is accurate. Once this has been achieved and the patient’s refraction is stable, a final irradiation step locks in the power change and IOL power.
Patients will continue to present to ODs asking about what new technologies are available in cataract surgery and the benefits they may provide. It is our role as eye care providers to educate them on surgical options such as FLACS and the many new IOL options, including the current research that suggests they have safe, efficient and reproducible track records thus far. We must guide patients with appropriate information to help them make an informed decision on what correction will suit them best.
Dr. Schweitzer is a cornea, glaucoma, cataract and refractive surgery specialist at Vance Thompson Vision in Sioux Falls, SD.
|
1. Nagy ZZ. New technology update: femtosecond laser in cataract surgery. Clinical Ophthalmology. 2014;8:1157-67. 2. Wu BM, Williams GP, Tan A, Mehta JS. A comparison of different operating systems for femtosecond lasers in cataract surgery. J Ophthalmol. 2015;2015:616478. 3. Sanders DR, Higginbotham RW, Opatowsky IE, Confino J. Hyperopic shift in refraction associated with implantation of the single-piece Collamer intra-ocular lens. J Cataract Refract Surg. 2006;32:2110-2. 4. Wallace RB 3rd. Capsulotomy diameter mark. J Cataract Refract Surg. 2003;29:1866-8. 5. Ravalico G, Tognetto D, Palomba M, et al. Capsulorhexis size and posterior capsule opacification. J Cataract Refract Surg. 1996;22:98-103. 6. Walkow T, Anders N, Pham DT, Wollensak J. Causes of severe decentration and subluxation of intraocular lenses. Graefes Arch Clin Exp Ophthalmol. 1998;236:9-12. 7. Cekic O, Batman C. The relationship between capsulorhexis size and anterior chamber depth relation. Ophthalmic Surg Lasers. 1999;30:185-90. 8. Erickson P. Effects of intraocular lens position errors on postoperative refractive error. J Cataract Refract Surg. 1990;16:305-11. 9. Baumeister M, Buhren J, Kohnen T. Tilt and decentration of spherical and aspheric intraocular lenses: effect on higher-order aberrations. J Cataract Refract Surg. 2009;35:1006-12. 10. Buckhurst PJ, Wolffsohn JS, Naroo SA, Davies LN. Rotational and centration stability of an aspheric intraocular lens with a simulated toric design. J Cataract Refract Surg. 2010;36:1523-28. 11. Toto L, Mastropasqua R, Mattei PA, et al. Postoperative IOL axial movements and refractive changes after femtosecond laser-assisted cataract surgery versus conventional phacoemulsification. J Refract Surg. 2015;31(8)524-30. 12. Richard J, Hoffart L, Chavane F, et al. Corneal endothelial cell loss after cataract extraction by using ultrasound phacoemulsification versus a fluid-based system. Cornea. 2008;27:17-21. 13. Krarup T, Holm LM, la Cour M, Kjaerbo H. Endothelial cell loss and refractive predictability in femtosecond laser-assisted cataract surgery compared with conventional cataract surgery. Acta Ophthalmologica. 2014;92(7)617-22. 14. Abell RG, Kerr NM, Howie AR, et al. Effect of femtosecond laser-assisted cataract surgery on the corneal endothelium. J Cataract Refract Surg. 2014;40(11):1777-83. 15.Popovic M, Campos-Moller X, Schlenker MB, et al. Efficacy and safety of femtosecond laser-assisted cataract surgery compared with manual cataract surgery. Ophthalmology. 2016;123(10):2113-26. 16. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2106;123(1):178-82. 17. Hoffmann PC, Hutz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36:1479-85. 18. Hayashi K, Manabe SI, Yoshida M, Hayashi H. Effect of astigmatism on visual acuity in eyes with a diffractive multifocal intraocular lens. J Cataract Refract Surg. 2010;36:1323-9. 19. Visser N, Bauer N, Nuijts R. Toric intraocular lenses: Historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39:624-37. 20. Soda M, Yaguchi S. Effect of decentration on the optical performance in multifocal intraocular lenses. Ophthalmologica. 2012;227:197-204. 21. Doane J. C&C CrystaLens AT-45 accommodating intraocular lens. Presented at the 20th Congress of the ESCRS, September, 2002; Nice, France. 22. Ong HS, Evans JR, Allan BD. Accommodative intraocular lens versus standard monofocal intraocular lens implantation in cataract surgery. Cochrane Database Syst Rev. 2014;5:CD009667. 23. Tecnis Symfony IOL. Abbott Medical Optics. 2016. Available at www.tecnisiol.com/eu/tecnis-symfony-iol.htm. Accessed October 31, 2016. 24. Hermann MM, Ustundag C, Diestelhorst M. Compliance with topical therapy after cataract surgery using a new microprocessor-controlled eye drop monitor. Invest Ophthalmol Vis Sci. 2005;46:e-abstract 3832. 25. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-142. 26. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39(1):8-14. 27. Lundstrom M, Albrecht S, Nilsson M, Anstrom B. Benefit to patients of bilateral same-day cataract extraction: randomized clinical study. J Cataract Refract Surg. 2006;32:826-30. 28. Leivo T, Sarikkola AU, Uusitalo RJ, et al. Simultaneous bilateral cataract surgery: economic analysis; Helsinki Simultaneous Bilateral Cataract Surgery Study report 2. J Cataract Refract Surg. 2011;37:1003-8. 29. Serrano-Aguilar P, Ramallo-Farina Y, Cabrera-Hernandez JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38(10):1734-42. 30. Jivrajka RV, Shammas MC, Shammas HJ. Improving the second-eye refractive error in patients undergoing bilateral sequential cataract surgery. Ophthalmology. 2012;119:109. |

