 |
A 78-year-old woman presented for an emergency visit with a chief complaint of “dimming” vision in both eyes of five days’ duration. She explained that things didn’t go black, just blurred, and now she required a handheld magnifier to see small print.
Her ocular history was positive for bilateral pseudophakia with bilateral YAG capsulotomies and open-angle glaucoma, surgically controlled via a combination of lens extraction and stent insertion. Her systemic history was positive for well-controlled hypertension and anemia. She was not diabetic. She denied trauma or allergies of any kind.
Clinical Findings
The patient’s best uncorrected entering visual acuities were 20/30 OD and 20/200 OS at distance and near with no improvement upon pinhole. A refraction of +0.50/+3.50 improved visual acuity to 20/25 OD and there was no improvement OS. Her external examination was normal OD and there was no afferent defect. Confrontation visual field OS revealed a central distortion covering the central 5-7 degrees.
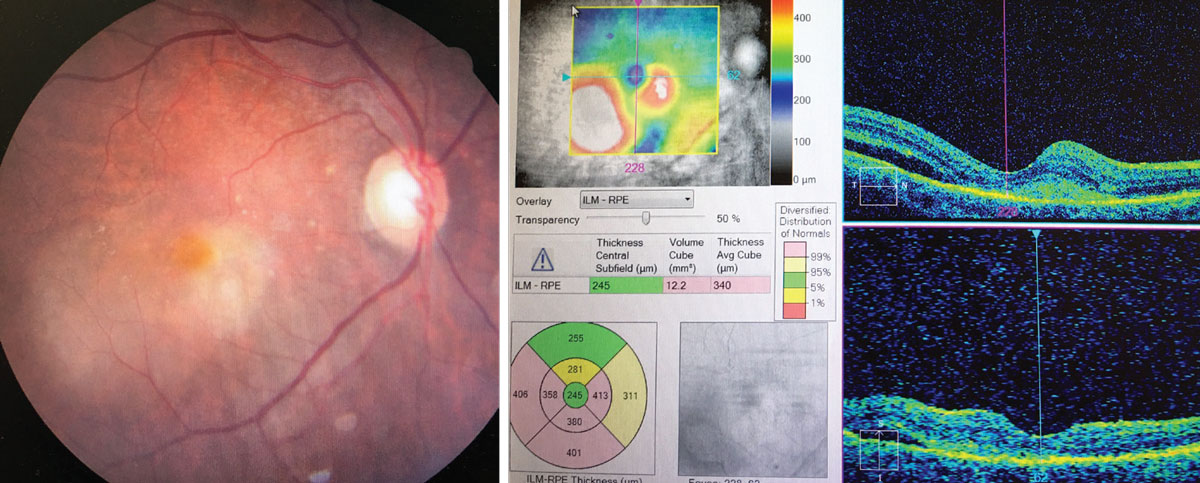
Biomicroscopic examination of the anterior segment found normal tissues and open angles. Her intraocular lenses were well centered and stable. Her intraocular pressures measured 12mm Hg OD and 16mm Hg OS using Goldmann applanation tonometry. The pertinent dilated fundus examination findings are demonstrated in the photo.
Additional Testing
Amsler grid testing was completed, demonstrating central blur and distortion. Brightness testing and color testing were completed, demonstrating no loss of either. Ocular photography was completed along with OCT. Carotid auscultation and blood pressure were also completed.
 |
|
This is the patient’s presentation and OCT. Are there any key findings that align with the patient’s report? Click image to enlarge. |
Diagnosis
This month’s case is an example of peripapillary choroidal neovascular membrane (CNV) OS as a result of dry age-related macular degeneration (AMD) conversion to wet AMD.
There are three classifications of CNV, each with the potential for having well-defined borders upon fluorescein angiography (classic) or not having well defined borders (occult):1,2
- Type 1 (typically occult) CNV remains beneath Bruch’s membrane but causes leakage of serous fluid beneath the retinal pigmented epithelium (RPE).
- Type 2 (typically classic) CNV is defined as CNV which penetrates Bruch’s membrane, typically causing severe fluid leakage into the retinal and subretinal structures.
- Type 3 CNV, known as retinal angiomatous proliferation (RAP), occurs when capillary proliferation develops within the retina.
CNV often forms in response to outer retinal ischemia, usually from compromised RPE function.3 These novel vascular structures are prone to leakage and can cause macular edema, pigment epithelial detachment (PED) or a serous retinal detachment, often with severe visual loss.4,5
Choroidal neovascularization is the most common source of severe vision loss in AMD.3-6 The disease is most common in white populations, with a prevalence of 6.8% in those over age 40 causing 54% of visual impairment in this population.3,7 AMD initially presents with subtle mottling of the RPE and the accumulation of drusen.4 Drusen are focal thickenings of Bruch’s membrane that produce elevations of the RPE; they are composed of lipid (lipofuscin), amyloid and complement factors.4 The accumulation of drusen and other cellular waste products disrupts the function of the RPE by impeding the flow of nutrients and oxygen to the RPE and retina, leading to ischemic conditions.1,4
As the disease advances, retinal ischemia causes the secretion of angiogenic cytokines such as vascular endothelial growth factor (VEGF). This can lead to exudative AMD, which is defined by the formation of a choroidal neovascular membrane.4,5 CNV may also occur in conditions that cause defects in Bruch’s membrane such as degenerative myopia, lacquer cracks, inflammatory conditions, choroidal rupture and ocular histoplasmosis syndrome.4
Type 1 CNV often requires imaging in the form of OCT or fluorescein angiography (FA) for positive identification. These cases may begin with PED alone or in synchronicity with bleeding, producing retinal elevation and sub-, intra- or preretinal serous fluid.
Type 2 CNV have greater potential for prominent subretinal or intraretinal fluid and hemorrhage.3
Fluorescein angiography (FA), OCT and indocyanine green angiography (ICG) can all be used for baseline assessment of CNV.1 FA is helpful to define the dimensions of the CNV and is necessary to classify the type of CNV lesion and to guide treatment when laser photocoagulation is used.1 OCT is helpful for identifying PED and evaluating the amount of serous fluid present as well as its location. Serial OCTs are often used to monitor the amount of edema to determine the efficacy of ongoing treatment.4 OCT angiography (OCT-A) can also be used to visualize CNV lesions with excellent detail although it cannot detect leakage.8
ICG makes use of the infrared spectrum of light and is used on lesions that cannot be well defined by FA and also for better visualization of the choroidal vasculature. It is the best tool for diagnosing polypoidal choroidal vasculopathy (PCP), a variant of AMD found more often in Black populations.4 PCP appears as a branching network of choroidal vessels with vascular dilations at the borders of the lesion.4
Choroidal neovascularization is often the cause of rapid vision loss, metamorphopsia or a central scotoma formation.3,4 If left untreated, chronic subretinal fluid and intraretinal macular edema cause photoreceptor death and catastrophic vision loss from large-scale RPE and retinal destruction. Eventually the leakage resolves, leaving a fibrous lesion in the macular area known as a disciform scar.
Management Options
Prompt treatment is recommended upon discovery of active CNV. The standard of care in almost all circumstances is intravitreal injection of anti-VEGF agents.3,4 Treatment is expected to produce stabilization of vision loss in 88.4% to 93.3% of patients and improvement of at least 15 letters in 25% to 34%.4 Visual acuity outcomes after two years have been found to be similar in “treat and extend” regimens when compared to monthly treatment regimens.4
Retinal laser photocoagulation has been used in the past but is now relegated to a small role due to the success of anti-VEGF agents and its known propensity to destroy tissue, increase lesion size and stimulate lesion re-growth. Occasionally, small CNV lesions outside the macula may be treated with focal laser without risk of causing vision loss.3,4
Photodynamic therapy has also been studied for treatment of CNV due to its safety compared to traditional laser photocoagulation. However, it is notably less effective than anti-VEGF agents.4
Peripapillary CNV has characteristic differences when compared to macular CNV. The effect on vision is more variable and the response to treatment with anti-VEGF agents is notably reduced. Treatment decisions are generally made on a case by case basis as there are no widely accepted treatment guidelines for peripapillary CNV.9
Polypoidal choroidal vasculopathy is less responsive to anti-VEGF treatments and is usually treated with photodynamic therapy.3
Ongoing monitoring of patients with CNV in one eye is important as there is a 15%/year risk of occurrence in the fellow eye.1 Vision loss from CNV can severely impair visual function and lead to disability and depression.1,3 Low vision aids and vision rehabilitation services may be of great use to patients suffering from severe vision loss from CNV (>20/200 or worse). Low vision devices such as magnifiers, reading lamps, closed-circuit televisions, typoscopes, motility assistance, contrast aids and other non-optical services may be of great benefit for specific tasks relating to better vision and quality of life.3
Our patient was promptly referred to the retina specialist, who confirmed the diagnosis and treated her with anti-VEGF injections.
Dr. Gurwood thanks Nick Karbach, OD and Denise Gurwood, OD, for their contributions to this case.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
|
1. Do DV. Detection of new-onset choroidal neovascularization. Curr Opin Ophthalmol. 2013;24(3):244-7. 2. Olaru A, Olaru DG, Nicolcescu A, et al. Retinal angiomatous proliferation case report. Rom J Ophthalmol. 2015;59(3):167-71. 3. Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379(9827):1728-38. 4. Nunes, RP, Rosenfeld PJ, de Amorim Garcia Filho CA, et al. Age-related macular degeneration. In: Yanoff M, Duker JS. Ophthalmology, 4th edition. Saunders: Elsevier, 2014;580-99. 5. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration [published correction appears in N Engl J Med. 2008 Oct 16;359(16):1736]. N Engl J Med. 2008;358(24):2606-17. 6. de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355(14):1474-85. 7. Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533-40. 8. Faatz H, Farecki ML, Rothaus K, et al. Changes in the OCT angiographic appearance of type 1 and type 2 CNV in exudative AMD during anti-VEGF treatment. BMJ Open Ophthalmol. 2019;4(1):e000369. 9. Jutley G, Jutley G, Tah V, et al. Treating peripapillary choroidal neovascular membranes: a review of the evidence. Eye (Lond). 2011;25(6):675-81. |

