The obligate intracellular parasite Toxoplasma gondii (T. gondii) is one of the most common causes of posterior uveitis in the human population.1-5 The most common ocular manifestation is a single or multifocal retinochoroiditis.
Let’s take a close look at a case of ocular toxoplasmosis as we provide an overview of the condition—including appropriate laboratory testing, diagnostic imaging and management strategies.
History
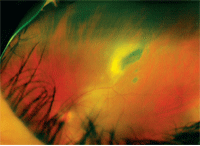
1. Fundus photograph of the patient’s left eye illustrated a 4in x 2in disc
diameter area of retinal edema that was associated with vasculitis and vitritis.
A 33-year-old Costa Rican male presented for an urgent eye exam. The patient never had a previous examination, and his chief complaint was decreased vision in his left eye. Also, he reported seeing approximately four to five large black spots in the vision of his left eye.
His symptoms began two weeks prior to the exam. He had not experienced any pain or flashes of light. But, the patient did note that he occasionally experienced headaches when moving from well lit to dimly lit rooms. He did not think that his vision had changed significantly since the onset of his symptoms and reported wearing sunglasses whenever he went outside.
His medical record showed that the patient had visited his primary care doctor for a wellness exam about eight months earlier. At that visit, a complete sexually transmitted disease (STD) panel was ordered as well as a tuberculosis (TB) screening. All STD testing came back negative; however, the patient had a positive purified protein derivative (PPD) test. The patient was promptly referred to the local TB clinic but failed to attend.
Diagnostic Data
Entering distance visual acuity was 20/20- O.D. and 20/20-2 O.S. Uncorrected near visual acuity was 20/20 O.U. The patient’s cover test, color testing, extraocular muscles and confrontation fields were unremarkable. His pupils were round and reactive to light, without relative afferent defect. A subjective refraction revealed negligible error.
Slit lamp examination revealed a grade 1+ anterior chamber reaction with corresponding mild keratic precipitates on the corneal endothelium in his left eye. There were also clumps of pigment on the anterior lens capsule of his left eye that were potentially indicative of a broken posterior synechia from a previous inflammatory event. The iris was fully motile at this visit, with no evidence of posterior synechia.
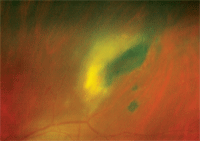
2. Magnified view of the lesion shown above.
Intraocular pressure measured 20mm Hg O.D. and 24mm Hg O.S. The patient denied symptoms of productive cough, fever, malaise or known exposure to anyone who was ill prior to the onset of his symptoms. He was not taking any medications and reported no known drug allergies.
The dilated fundus examination was unremarkable O.D. However, the left eye revealed a moderate vitritis that slightly obscured our view of the retina. Further, a white, fluffy, elevated lesion representing an active retinitis was documented just outside the superior temporal vascular arcade (figures 1 and 2). An area of vascular sheathing with an associated intraretinal hemorrhage was located next to the lesion. There was no evidence of inflammation involving either optic nerve head or macula.
We immediately referred the patient to a retinologist for a complete evaluation. Because of his recent history of a positive PPD test, we could not rule out an active TB lesion. Based on the retinal presentation and history, the retinologist diagnosed our patient with ocular toxoplasmosis. We initiated a prompt course of oral and topical medications prior to any confirmatory blood work, including 1 tab Bactrim (sulfamethoxazole and trimethoprim, Mutual Pharmaceutical) b.i.d.; 1,000mg clindamycin q.i.d.; 40mg Prilosec (omeprazole, AstraZeneca) q.d.; 40mg prednisone q.d.; Pred Forte (prednisolone acetate, Allergan) q4h O.S.; and Cyclogyl (cyclopentolate hydrochloride, Alcon) t.i.d., O.S.
One week later, our patient presented to the retinal specialist for a follow-up. The retinal specialist noted that the condition was improving and that the active area of inflammation was decreased in size. The course of treatment was left unchanged, and scheduled the patient for another one-week follow-up examination. Unfortunately, the patient failed to follow-up after this visit. Repeated attempts to contact the patient by phone and mail were unanswered.
Discussion
Toxoplasma gondii is a protozoan parasite that is predominantly hosted by felines; however, any mammal or bird may serve as an intermediate host. Toxoplasmosis infection can either occur congenitally or be acquired throughout adulthood. Its seroprevalance increases among individuals of all races with advanced age.6 In healthy, immunocompetent patients, infection can pass with only mild, flu-like symptoms that include fever, malaise and myalgia. But, in patients who are immunocompromised (especially those with HIV who exhibit CD4 counts below 200 cells/µL), infection can cause encephalitis, myocarditis and pneumonitis––ultimately leading to death.
Infection of the host occurs when the feline ingests any one of the three forms of T. gondii: the rapidly replicating tachyzoite, the quiescent bradyzoite, or the oocytic, which is the fertilized zygote.7 It is here, inside the definitive host, where the sexual reproductive cycle occurs. The male microgametes fertilize the female macrogametes to form oocysts that are shed in the cat’s feces to undergo maturation outside the feline intestine.7 These oocysts can be found in the feces for seven to 20 days after infection. The oocysts are not infectious immediately, but rather become infectious after one to four days at room temperature. They can remain infectious for up to one year in the soil. The oocysts can be destroyed by freezing or heating to 70°C.5
Upon ingestion by a human, the cyst wall is broken down in the intestinal tract, releasing the bradyzoites, which permits the parasite to enter the intestinal wall.7,8 The bradyzoites are activated and become rapidly multiplying tachyzoites.7,8 The tachyzoites then invade host tissues—specifically the muscle and nervous tissues—and revert back to their quiescent bradyzoite form, completing the asexual reproductive cycle.7,8 In immunocompetent patients, the encysted bradyzoites can remain dormant, failing to incite the host immune response.7 However, in immunocompromised patients, spontaneous reactivation can occur and cause repeated tissue damage or death (if left untreated).8
So, how do humans acquire this infection? It is transmitted via direct contact with contaminated food, soil, sand or cat litter and often occurs following the consumption of raw or undercooked meat. Eating undercooked meats and contact with cat feces has been correlated with toxoplasmosis antibodies in Costa Ricans.9 Several other studies have suggested that infections can be acquired through contaminated water, blood transplants and organ donations.10-14 The infection can also be passed on from mother to fetus through the placental circulation, resulting in a congenital infection. (figure 3).
Prevalence and Prevention
Human infection can occur congenitally or through direct contact. Congenital infection can occur at any stage during pregnancy, but is most common during the third trimester.3 An estimated 50% of untreated infections are passed onto the fetus during pregnancy.10 The most common manifestation of congenital infection is retinochoroiditis. Due to its propensity to involve the macula, congenital lesions tend to be more visually devastating than the acquired disease form, which chiefly produces peripheral lesions.3,16,17
Many congenital infections could be prevented; however, most pregnant women remain unaware of the associated risk factors.18 Recommendations to prevent infection during pregnancy include avoiding raw or undercooked meats, washing fruits and vegetables, washing all cooking surfaces that come into contact with raw meats, fruits and vegetables, avoiding changing cat litter, and wearing gloves when working in soil or sand.15
Due to the ubiquitous nature of T. gondii and the preventability of infection, several countries have continued efforts to identify the prevalence of the organism as well as at-risk populations. According to the most recent data published by the third National Health and Nutrition Examination Survey (NHANES), the overall age-adjusted seroprevalence in the
United States was 22.5%.6 The seroprevalence for women of child-bearing age (15 to 44 years) was 15%.6 The survey also found a higher seroprevalence in Northeastern and Southern U.S. localities.6
Other risk factors for infection include contact with foreign-born citizens, individuals of lower education levels, those who live in crowded environments and laborers who frequently work in soil.6
Prevalence in the U.S. remains relatively low compared to other countries. One study completed in Costa Rica found that the seroprevalence was 76%, and a second unrelated study found the seroprevalence to be 61.4%.9,19 Nonetheless, both studies indicated that higher infection rates were directly proportional with increased consumption of raw or undercooked meats and cat exposure.9,19 Other studies have found a high seroprevalence in France, Latin American, sub-Saharan Africa and Brazil.6,20
Laboratory Testing
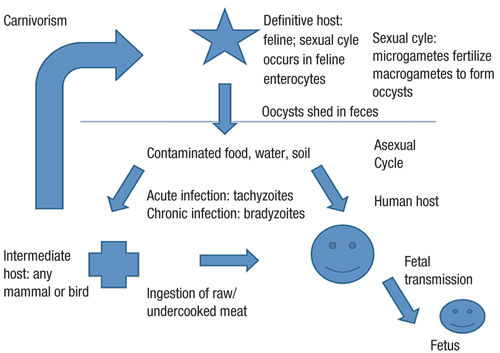
3. A general schematic of the T. gondii life cycle.
Determining the presence of T. gondii infection is done in one of three ways: isolation of the actual organism, isolation of the organism’s DNA, or isolation of the specific antibodies that are activated by the organism.21,22
• Isolating the organism. This involves obtaining a sample of whole blood or other body fluids and inoculation of a mouse.21,22 The detection of the parasite in these specimens indicates an acute infection.21,22
• Isolating the organism’s DNA. The presence of T. gondii DNA is determined by polymerase chain reaction (PCR) amplification from a urine specimen or a sample of amniotic, peritoneal, pleural, ocular (vitreous or aqueous) or cerebrospinal fluids.21,22 PCR has been proven valuable in immunocompromised patients due to the decreased antibody response to infection.21
Also, because amniotic fluid can be used to detect the presence of DNA, it can be used to test for congenital infections with minimal trauma to the fetus.22
• Isolating the organism’s antibodies. Measuring the levels of individual antibodies by serologic testing can differentiate between acute and chronic infections. The two most common antibodies tested are IgG and IgM.21,22 The FDA recommends the use of a confirmatory test in the case of suspected acute infections where treatment is considered.22,23
Anti-Toxoplasma IgM commercial test kits are available, but they often have low specificity.
Several conditions have a similar appearance to toxoplasmosis retinocoroiditis, including syphilis, tuberculosis, sarcoidosis and Toxacara cannis infection.21,24 These tests should be performed if the diagnosis remains in doubt:
• Fluorescent treponemal antibody absorption (to rule out syphilis).
• Purified protein derivative with anergy panel and chest X-ray (to rule out tuberculosis and sarcoidosis).
• Angiotensin converting enzyme (to rule out sarcoidosis)
• Toxocara ELISA (to rule out Toxocara cannis infection).
Because immune status plays a role in determining the course of treatment, testing for the presence of human immunodeficiency virus is also important for patients who are at risk for HIV as well as for atypical cases/presentations.21,24
Diagnostic Imaging
Diagnostic imaging includes intravenous fluorescein (IVF) angiography, indocyanine green (ICG) angiography, optical coherence tomography (OCT), B-scan ultrasonography and standard photography.21 IVF and ICG exhibit hyperfluorescence that becomes hypofluorescent as inflammation progresses to scarring.21 OCT, with its ability to highlight retinal edema and vitreoretinal interactions, is particularly useful in imaging the resolution of edema and the presence of newly formed epiretinal membranes or neovascular complications.21 In chronic infections where dense media opacification has occurred, standard B-scan ultrasonography can be employed to visualize the posterior segment and rule out retinal detachments.21 Finally, photo documentation can also be utilized to closely follow lesions for resolution.
Management
Toxoplasmosis is a self-limiting condition that can persist up to four months.24 Lesions that do not directly threaten the optic nerve or macula are not usually treated.24,25 Treatment is initiated when lesions affect the optic nerve or macula, cause a vitritis that results in at least a two-line decrease in visual acuity or significant vitreo-retinal traction, involve one of the larger retinal vessels or cause intravitreal and/or intraretinal hemorrhaging.25
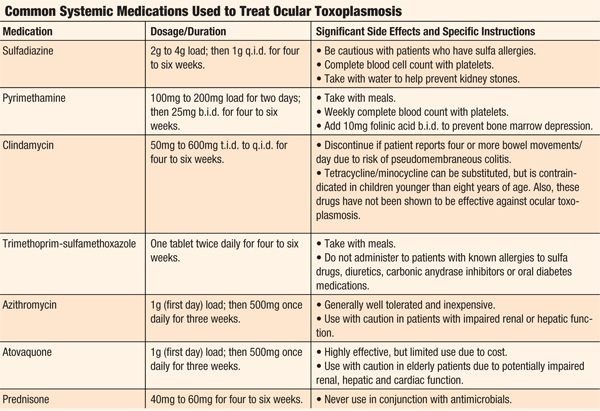
Current treatment includes oral antiparasitic drugs, such as triple-therapy clindamycin, sulfadiazine and pyrimethamine.24-26 When using pyrimethamine, be sure to include a folic acid supplement as well as obtain platelet counts on a weekly basis. Treatment is usually administered over a period of three to four weeks.3,27-29
The use of corticosteroids is controversial and should only be used in combination with the antiparasitic drugs.30 The management of any granulomatous uveitis can be accomplished with a combination of a topical cycloplegic and topical corticosteroids (the dosage should be dictated by the severity of the presentation). Due to the severe side effects of many of these medications, long-term suppressive therapy is reserved only for those patients who are significantly immunocompromised (see “Common Systemic Medications Used to Treat Ocular Toxoplasmosis”).21,24-26
In some cases, surgical intervention may be necessary. Panretinal photocoagulation is utilized in the presence of retina hypoxia.24,26 Cryopexy of active lesions may be effective due to T. gondii’s susceptibility to cold temperatures.24,26 A vitrectomy can be performed if there is dense, non-resolving vitreous opacification.24,26
Toxoplasma gondii is a ubiquitous organism in the environment, so you’ll likely encounter a patient with either active or quiescent lesions. When a patient presents with a granulomatous uveitis, a complete ocular examination––including a dilated fundus assessment––is vital to evaluate for posterior involvement.
Because toxoplasmosis is a chronic infection that may require treatment with potent antibiotics, prompt referral for systemic management with an infectious disease specialist helps minimize tissue damage and possible ocular morbidity.
Dr. Bauer works as a primary eye care provider at Open Cities Health Center in St. Paul, Minn. where he helped launch a full-time eye clinic that focuses on decreasing health disparities in the community. Dr. Dzurinko was an assistant clinical professor at the New England College of Optometry from 2008 to 2009, and is currently in private practice in Wilmington, Mass.
1. Tabbara KF. Ocular toxoplasmosis: toxoplasmic retinochoroiditis. Int Ophthalmol Clin. 1995 Spring;35(2):15-29.
2. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000 Nov;30(12-13):1217-58.
3. Rothova A. Ocular involvement in toxoplasmosis. Br J Ophthalmol. 1993 Jun;77(6):371-7.
4. Labalette P, Delhaes L, Margaron F, et al. Ocular toxoplasmosis after the fifth decade. Am J Ophthalmol. 2002 Apr;133(4):506-15.
5. Pavesio CE, Lightman S. Toxoplasma gondii and ocular toxoplasmosis: pathogenesis. Br J Ophthalmol. 1996 Dec;80(12):1099-107.
6. Jones JL, Kruszon-Moran D, Wilson M, et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001 Aug 15;154(4):357-65.
7. Baron S. Medical Microbiology 4th edition. Galveston, Texas: The University of Texas Press; 1996;84.
8. Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000 Sep;64(3):607-23.
9. Arias ML, Chincilla M, Reyes L, Linder E. Seroepidemiology of toxoplasmosis in humans: possible transmission routes in Costa Rica. Rev Biol Trop. 1996 Aug;44(2A):377-81.
10. Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, et al. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. 2003 Jan;9(1):55-62.
11. Bell A, Gill R, Isaac-Renton J, et al. Outbreak of toxoplasmosis associated with municipal drinking water—British Columbia. The British Columbia Toxoplasmosis Team. Can Commun Dis Rep. 1995 Sep 30;21(18):161-3.
12. Heukelbach J, Meyer-Cirkel V, Moura RC, et al. Waterborne toxoplasmosis, northeastern Brazil. Emerg Infect Dis. 2007 Feb;13(2):287-9.
13. Alvarado-Esquivel C, Mercado-Suarez MF, Rodríguez-Briones A, et al. Seroepidemiology of infection with Toxoplasma gondii in healthy blood donors of Durango, Mexico. BMC Infect Dis. 2007 Jul 13;7:75.
14. Centers for Disease Control and Prevention. Parasites: Toxoplasmosis. Available at:
www.cdc.gov/parasites/toxoplasmosis/ (accessed November 23, 2010).
15. Lopez A, Dietz VJ, Wilson M, et al. Preventing congenital toxoplasmosis. MMWR Recomm Rep. 2000 Mar 31;49(RR-2):59-68.
16. Kodjikian L, Wallon M, Fleury J, et al. Ocular manifestations in congenital toxoplasmosis. Graefes Arch Clin Exp Ophthalmol. 2006 Jan;244(1):14-21.
17. Atmaca LS, Simsek T, Batioglu F. Clinical features and prognosis in ocular toxoplasmosis. Jpn J Ophthalmol. 2004 Jul-Aug;48(4):386-91.
18. Jones JL, Ogunmodede F, Scheftel J, et al. Toxoplasmosis-related knowledge and practices among pregnant women in the United States. Infect Dis Obstet Gynecol. 2003;11(3):139-45.
19. Frenkel JK, Ruiz A. Human toxoplasmosis and cat contact in Costa Rica. Am J Trop Med Hyg. 1980 Nov;29(6):1167-80.
20. Sobral CA, Amendoeira MR, Teva A, et al. Seroprevalence of infection with Toxoplasma gondii in indigenous Brazilian populations. Am J Trop Med Hyg. 2005 Jan;72(1):37-41.
21. Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005 Jul-Sep;20(3):129-41.
22. Remington JS, Montoya JG. Palo Alto Medical Foundation. Toxoplasma Serology Laboratory. Available at:
www.pamf.org/serology/clinicianguide.html (accessed November 23, 2010).
23. Burlington B. FDA Public Health Advisory: Limitations of Toxoplasma IgM Commercial Test Kits. Available at:
www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062411.htm (accessed November 23, 2010).
24. Alexander LJ. Primary Care of the Posterior Segment. Columbus, Ohio: McGraw-Hill Medical; 2002.
25. Bartlett JD, Jaanus SD. Pocket Companion to Clinical Ocular Pharmacology. Philadelphia: Elsevier Science; 2002.
26. Koo L, Young LH. Management of ocular toxoplasmosis. Int Ophthalmol Clin. 2006 Spring;46(2):183-93.
27. Beverley JK. Toxoplasmosis. Br Med J. 1973 May 26;2(5864):475-8.
28. Tabbara KF, O’Connor GR. Treatment of ocular toxoplasmosis with clindamycin and sulfadiazine. Ophthalmology. 1980 Feb;87(2):129-34.
29. Borkowski PK. New trends in ocular toxoplasmosis—the review. Przegl Epidemiol. 2001;55(4):483-93.
30. Bosch-Driessen EH, Rothova A. Sense and nonsense of corticosteroid administration in the treatment of ocular toxoplasmosis. Br J Ophthalmol. 1998 Aug;82(8):858-60.

