 A 30-year-old Hispanic female presented complaining of difficulty seeing road signs while driving at night. She had never worn glasses and denied any history of eye problems. Her medical history was unremarkable.
A 30-year-old Hispanic female presented complaining of difficulty seeing road signs while driving at night. She had never worn glasses and denied any history of eye problems. Her medical history was unremarkable.
On examination, her best-corrected visual acuity measured 20/20 O.U., with a low myopic correction. Confrontation visual fields were full to careful finger counting O.U. Pupils were equally round and reactive, with no evidence of afferent defect. Ocular motility testing was normal. The anterior segment examination was unremarkable O.U. Her intraocular pressure measured 13mm Hg O.U.
Dilated fundus exam revealed a clear vitreous O.U. The optic nerves appeared healthy, with healthy rim coloration and perfusion. We noted changes in the posterior poles of both eyes (figures 1 and 2). We also ordered a spectral-domain optical coherence tomography (SD-OCT) scan (figures 3 and 4).
Take the Retina Quiz
1. What do the fundus changes in the posterior poles represent?
a. Drusen.
b. Lipofuscin.
c. Exudate.
d. Roth spots.
2. What does the SD-OCT show?

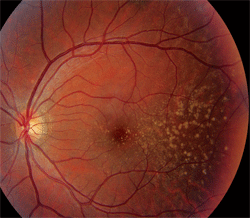
1,2. Fundus photographs of the right and left eye of our patient (O.D. left, O.S. right) show obvious changes throughout the posterior poles.
a. Polypoidal aneurysmal dilation.
b. Choroidal neovascularization (CNV).
c. Nodular thickening of the retinal pigment epithelium (RPE) and Bruch’s membrane.
d. Lipofuscin storage at the level of Bruch’s membrane.
3. What is the correct diagnosis?
a. Basal laminar drusen.
b. Polypoidal choroidal vasculopathy.
c. Macular degeneration.
d. Stargardt’s macular dystrophy.
4. How should this patient be managed?
a. Observation.
b. AREDS-formulated nutraceuticals as well as lutein and zeaxanthin supplements.
c. Intravitreal Avastin (bevacizumab, Genentech) injection.
d. Photodynamic therapy (PDT).
5. Would you recommend genetic testing?
a. Yes.
b. No.
For answers, see below.
Discussion
The changes seen in our patient represent drusen formation. Of course, the important question is: Why does a 30-year-old patient have drusen? Does she have macular degeneration? If she exhibited this presentation in her 60s, AMD would be a very reasonable diagnosis. But, 30-year-old individuals simply do not develop macular degeneration.
Instead, our patient has basal laminar drusen, which is also sometimes referred to as cuticular drusen. These represent nodular thickening of the RPE’s basement membrane––as opposed to the type of drusen that are caused by AMD, which result from focal detachments of a normal-thickness basement membrane.
Basal laminar drusen typically are seen in early adulthood, and exhibit no racial predilection. The drusen often appear as discretely round, slightly raised, yellow, subretinal nodules that initially may be randomly scattered throughout the macular area of young adults, but become more numerous over time. These basal laminar drusen usually are grouped in clusters of 15 to 20. Typically, the clusters are arranged in a tightly knit pattern, giving the entire macula and posterior pole an “orange peel” appearance.
In our patient, the drusen seem to be localized to the temporal aspect of the macula and radiate in an almost triangular fashion. Some drusen involve the macula, however. Nonetheless, as our patient ages, the number of drusen likely will increase gradually.
Basal laminar drusen is a diagnosis based on the appearance of clinical features, which are easily identified on fundoscopy. Additional testing is often unnecessary, unless there is suspicion of CNV. In these instances, fluorescein angiography (FA) has been used to help identify areas of active leakage from the CNV. Also, FA yields a very characteristic staining pattern, which is described as a “stars-in-the-sky” or “milky-way” appearance.
In recent years, SD-OCT has largely supplanted FA due to its noninvasive nature and high-resolution imaging capabilities, which provide incredible cross-sectional detail of the retinal anatomy. This is highlighted in our patient, where we can see the areas of nodular thickening and irregularities of the RPE. We performed SD-OCT on our patient because we wanted to obtain a baseline image that could be used for comparison during future examinations––not because we suspected CNV.
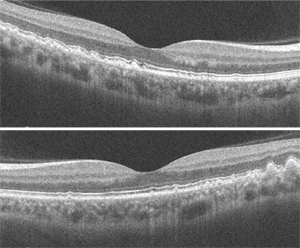
3, 4. SD-OCT of our patient’s right (top) and left eye. How would you interpret the scans?
So, how should our patient be managed? Could there be any value in recommending an AREDS-formulated supplement? AREDS underscored the value of nutritional supplementation in patients with macular degeneration who were at high risk for the development of CNV.1 But, would nutritional supplementation apply to our patient? Technically, she doesn’t have AMD.
Given the changes seen in the patient’s macula, you can’t help but think that, at some point in her lifetime, the risk for developing more severe degenerative changes would increase. Indeed, according to J. Donald M. Gass, M.D., patients over the age of 50 who are susceptible to developing superimposed, exudative drusen in the center of their maculae, are also likely to develop CNV.2
But unfortunately, no clear scientific evidence supports the use of nutritional supplements in patients with basal laminar drusen. Intuitively, however, it is reasonable to believe that nutritional supplements that contain lutein and zeaxanthin and/or omega-3 fatty acids would offer some protective benefit in these cases.
What about genetic testing for our patient? MaculaRisk (ArcticDx) is the first commercially available DNA test for AMD. MaculaRisk categorizes people into five levels of risk assessment based on specific genetic markers associated with macular degeneration. The test has an 83% probability of successfully identifying early AMD patients who will progress to exudative AMD. The cost is approximately $750. Most insurance carriers cover the expense as long as there are clinically relevant features, such as drusen or RPE mottling.
However, a MaculaRisk assessment may not be applicable for our patient. Interestingly, the genetic markers for AMD that are used by this test are chiefly based on patients of white European descent. Currently, the necessary scientific information to interpret the genetic risk of AMD for patients of other ethnicities is unavailable. Because our patient is Hispanic, MaculaRisk may not be a legitimate consideration. But, it would be both interesting and clinically useful to know her genetic predisposition for advanced AMD development.
We explained the findings to our patient. I recommended an AREDS-formulated supplement that included lutein, zeaxanthin and omega-3 fatty acids. We gave the patient a home Amsler grid and instructed her to return should she notice any changes. For now, we elected to follow her on a yearly basis.
1. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36.
2. Gass JD. Stereoscopic atlas of macular disease: diagnosis and treatment. 4th ed. St Louis: Mosby; 1997.
Answers to the Retina Quiz
1. a
2. c
3. a
4. b
5. b

