 A 62-year-old Hispanic male presented for an eye exam at the request of his physician. He is HIV-positive and has hepatitis C (HCV). The patient reported that he experienced no difficulties with his vision and that he has not had an eye examination for five years. He currently uses over-the-counter reading glasses for near vision correction.
A 62-year-old Hispanic male presented for an eye exam at the request of his physician. He is HIV-positive and has hepatitis C (HCV). The patient reported that he experienced no difficulties with his vision and that he has not had an eye examination for five years. He currently uses over-the-counter reading glasses for near vision correction.
With regards to his HIV, he claims his viral load is undetectable and his CD4 count is in the 300s. The patients physician started him on two new HCV medications approximately six weeks earlier: Pegasys (peginterferon alpha-2a, Roche Laboratories) and ribavirin.
The patient also takes Norvir (ritonavir, Abbott Laboratories), Reyataz (atazanavir sulfate, Bristol-Myers Squibb) and Epzicom (abacavir sulfate and lamivudine, GlaxoSmithKline) for his HIV and Wellbutrin XL (bupropion, GlaxoSmithKline) for depression.
On examination, his best-corrected visual acuity was 20/20 O.U. at distance and near. Confrontation visual fields were full to careful finger counting O.U.
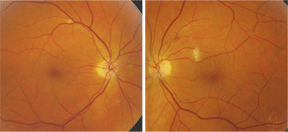
His pupils were equally round and reactive, with no afferent pupillary defect. His extraocular motility testing was normal. The anterior segment exam was unremarkable. Intraocular pressure measured 13mm Hg O.U. by applanation tonometry. The dilated fundus exam revealed a clear vitreous in each eye. He had small cups with good rim coloration and perfusion O.U. The vessels were of normal caliber O.U., and each macula appeared normal with a good foveal light reflex. The peripheral retina was normal O.U. Note the changes in both eyes, as seen in figures 1 and 2.
1, 2. Fundus images of a 62-year-old patient with HIV and hepatitis C (O.D. left, O.S. right). What finding do the pictures reveal?
Take the Retina Quiz
1. What do the white lesions in both eyes represent?
a. Exudate.
b. Cotton-wool spots.
c. Focal areas of retinitis.
d. Serous fluid.
2. What additional testing is warranted for this patient?
a. In-office blood pressure.
b. Complete blood count and fasting blood glucose.
c. Both a and b.
d. No additional testing is necessary at this time.
3. What is the most likely diagnosis?
a. Chronic anemia.
b. HIV microangiography retinitis.
d. Interferon retinopathy.
4. How should this patient be managed?
a. Inform his physician of the ocular findings.
b. Follow-up with the patient on a weekly basis.
c. Follow-up with the patient every four to six weeks.
d. Both a and c.
For answers, see below.
Discussion
The white fundus lesions seen in our patients eyes represent cotton-wool spots. A small spot can be seen along the superior arcade in the right eye. Also, two smaller spots, which appear to be resolving, can be seen nasal and inferior to the optic nerve O.D.
In the left eye, a larger cotton-wool spot is present adjacent to the macula. There is also a flame-shaped hemorrhage superior to the optic nerve O.S. These changes represent microangiopathy. They are not due to HIV, but are probably a side effect of the interferon therapy for hepatitis C.
HCV is a virus that occurs by direct blood-to-blood contact with an infected individual.1 Nearly 200 million people have HCV worldwide, including four million Americans.2,3 Risk factors for HCV include unprotected sexual contact, intravenous drug abuse and multiple body piercings or tattoos.1
Patients commonly present with a co-infection of HIV and HVC. A Canadian study reported that 20% of HIV-positive patients were also infected with HCV.4 This is not tremendously surprising, considering that both groups share similar modes of infection.
Co-infection with HIV may increase the frequency and persistence of HCV. Additionally, there is less chance for HCV to resolve spontaneously in patients who are co-infected with HIV. Finally, patients who are infected with both HIV and HCV may experience faster progression to liver disease.1
The natural course of chronic HCV varies from person to person. Many patients with HCV are asymptomatic or have only mild symptoms and never seek treatment. Among untreated patients, up to 20% progress to liver cirrhosis in less than 20 years.1
Typical treatment for HCV includes a combination therapy of pegylated interferon alpha and ribavirin for 24 to 48 weeks.1,5 In the early 1990s, several reports surfaced of patients who developed interferon retinopathy.6,7 According to one report, the incidence of retinopathy widely varied between 18% and 86% of patients on interferon therapy.8
Intraretinal hemorrhages and cotton-wool spots are the most common findings associated with interferon retinopathy. Less common findings include optic disc hyperemia and macular edema.9 Rare complications include branch retinal artery occlusion, retinal detachment, vitreous hemorrhage, subconjunctival hemorrhages and disc edema.9 It is difficult to determine if these rare complications represent true associations or are purely coincidental. Most reported cases of interferon retinopathy have been mild and non-sight threatening.8,9
Interferon retinopathy usually develops between eight and 12 weeks after the initiation of treatment, and typically resolves within four to 12 weeks after discontinuation of therapy. The mechanism remains unknown, although ischemia and deposition of immune complexes (which lead to capillary nonperfusion) have been implicated.9 In most instances of interferon retinopathy, patients are able to continue therapy. If the physician decides to continue therapy, you should follow the patient closely.
We notified our patients primary-care provider about the findings. The physician was reluctant to discontinue the interferon because the patient responded very well to therapy. As a result, we decided to see the patient regularly in four- to six-week intervals for complete ocular evaluation.
Retina Quiz Answers: 1) b; 2) c; 3) d; 4) d
1. Hoofnagle JH, Lindsay KL. Acute Viral Hepatitis. In: Goldman L, Ausiello D (eds). Cecil Textbook of Medicine, 22nd ed.
2. Kieny MP. Initiative for vaccine research: hepatitis C. The World Health Organization. Available at: www.who.int/vaccine_research/diseases/hepatitis_c/en/ (Accessed November 11, 2008).
3. Leshner AI. Community drug alert bulletin: hepatitis. The National Institute on Drug Abuse (NIDA). Available at: www.drugabuse.gov/HepatitisAlert/HepatitisAlert.html (Accessed November 11, 2008).
4. Cote P, Baril JG, Hebert MN, et al. Management and treatment of hepatitis C virus in patients with HIV and hepatitis C virus coinfection: A practical guide for health care professionals. Can J Infect Dis Med Microbiol 2007 Sep;18(5): 293-303.
5. The Mayo Foundaiton for Medical Education and Research. Infectious disease: hepatitis C. Available at: www.mayoclinic.com/health/hepatitis-c/DS00097/DSECTION=treatments-and-drugs (Accessed November 11, 2008).
6. Ikebe T, Nakatsuka K, Goto M, et al. A case of retinopathy induced by intravenous administration of interferon. Folia Ophthalmol Jpn (Ganka-Kiyo) 1990;41:2291-6.
8. Hayasaka S, Nagaki Y, Matsumoto M, et al. Interferon associated retinopathy. Br J Ophthalmol 1998 Mar;82(3): 323-5.
9. Schulman JA, Liang C, Kooragayala LM, King J. Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmology 2003 Feb;110 (2):437-42.

