In recent years, management of glaucoma has changed considerably, adding an enhanced prostaglandin analog, an entirely new class of drugs and a new sustained-release delivery system. The discussion with patients now includes laser therapy as a first-line treatment option that diminishes concern for patient adherence and ocular surface disease. Every patient will have a unique approach; however, the diagnosis or risk of glaucoma should be definitive before starting or changing glaucoma treatment.
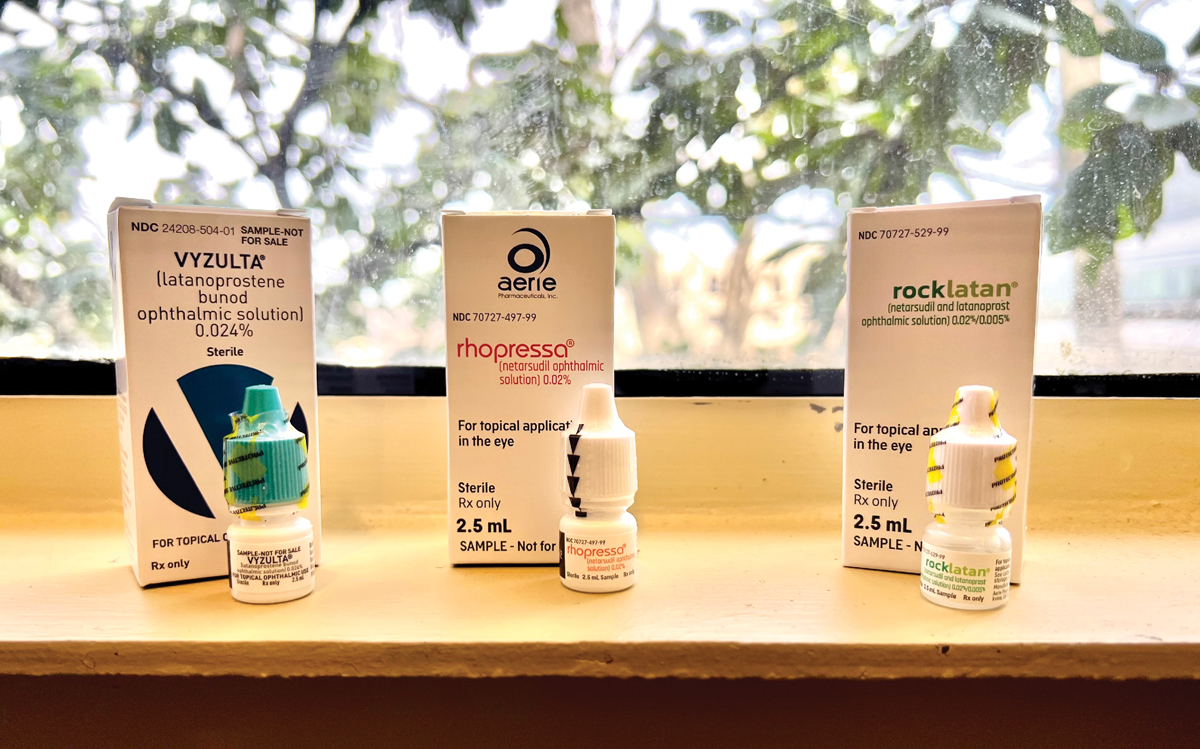 |
Vyzulta, Rhopressa and Rocklatan are novel drops to consider for glaucoma patients. Photo: Abagail Kirk, OD. Click image to enlarge. |
The Big Players
Recently published studies indicate long-term risk of glaucoma in ocular hypertensive patients. Approved by the FDA in 2017 for primary open-angle glaucoma (POAG) and ocular hypertension (OHT), Vyzulta (latanoprostene bunod 0.024%, Bausch + Lomb) is a topical ophthalmic solution made up of latanoprost, which promotes uveoscleral outflow, and butanediol mononitrate, which increases conventional outflow.1
Most glaucoma drops only alter uveoscleral outflow, but in a healthy eye the majority of outflow occurs via the trabecular meshwork (TM). Butanediol mononitrate donates nitric oxide, a molecule deficient in glaucomatous eyes, which relaxes the trabecular meshwork, thus increasing aqueous outflow.2
The VOYAGER study showed Vyzulta to be more effective than latanoprost 0.005% by ~1.23mm Hg when observing diurnal intraocular pressures (IOPs).3 Two noninferiority studies, LUNAR and APOLLO, established Vyzulta QD to be more effective than timolol 0.5% BID (32.0% compared to 27.6%).2
Vyzulta also has better 24-hour efficacy compared with timolol, which is crucial for elevated nocturnal IOP, indicated by the CONSTELLATION study.4 Large IOP fluctuations and high nocturnal IOP spikes are evident especially in progressive low-pressure glaucomas, and Vyzulta provides significant IOP lowering in normal-tension glaucoma.5 Side effects, similar to other prostaglandins, include mild hyperemia (16.7%), irritation (11.9%), eyelash changes (16.7%) and iris and periorbital tissue pigmentation (4.0% and 3.2%, respectively).4,6
Historically, prostaglandins have been prescribed first due to their highly effective yet safe profile. Up until now, combination drugs involving prostaglandin did not exist. With Vyzulta’s higher efficacy and once-daily dosing, patient adherence can improve, reducing risk of glaucomatous progression. Given these merits, Vyzulta serves as an excellent first-line therapy, but can also be used as second-line if desiring to avoid surgery.2
As stated above, the conventional outflow pathway is responsible for the majority of aqueous outflow. Similar to nitric oxide-donating drugs, rho-kinase (ROCK) inhibitors enhance outflow through the TM. This is accomplished by decreasing actin and myosin-driven cellular contraction and reducing extracellular matrix protein production.7 Additionally, ROCK inhibitors demonstrate neuroprotective potential in animal studies and improved anterior and posterior segment vessel density in humans, measured by OCT angiography.8-11
The first ROCK inhibitor to be approved in the United States was Rhopressa (netarsudil 0.02%, Aerie) in 2017. Beyond its ROCK inhibition, it has inhibitory action against norepinephrine transporter (NET), making it a ROCK/NET inhibitor. NET inhibition causes vessel constriction and reduction of blood flow to the ciliary processes, therefore reducing aqueous production and lowering IOP. Similarly, NET inhibition reduces episcleral venous pressure through vasoconstriction, accounting for greater than a third of netarsudil’s IOP lowering effect.12,13
Multiple Phase III studies (ROCKET 1, 2 and 4) have shown that netarsudil QD is at least equivalent to timolol 0.5% BID in patients with POAG and OHT. A 2015 study comparing various concentrations of netarsudil to latanoprost determined that netarsudil was ~1mm Hg less effective than latanoprost for patients with IOP of 22mm to 36mm Hg, but netarsudil was equally effective for patients with IOP below 26mm Hg. Accordingly, netarsudil may be more effective in patients with lower baseline IOPs and might be a preferred choice in the management of patients with lower pressure glaucomas.14
Across all studies, netarsudil has been shown to decrease IOP by between 20% and 40%, which is similar to currently recommended first-line therapies.15 Netarsudil’s range of use is broad, as it has recently proven to be effective in patients already on maximal medical therapy (average of three or more medications in this study) whose remaining options previously would have been surgical.16
Netarsudil is well-tolerated, with the most common adverse event being conjunctival hyperemia (54.4%). These vascular changes found in studies and in practice are not surprising given the established vasodilatory effects of ROCK inhibitors, distinct from NET inhibition vasoconstriction. Mild, reversible corneal verticillata (20.9%) and conjunctival hemorrhage (17.2%) were also noted.17 In summary, research validates the versatility of once-daily administration of netarsudil as a safe, convenient and effective medication across a wide spectrum of management needs.
In 2019, Rocklatan (Aerie) was introduced as a fixed-dose combination of netarsudil 0.02% and latanoprost 0.005%, dosed once nightly. Combining the two medications provides an option that has four mechanisms of action: increased uveoscleral and trabecular outflow, decreased aqueous production and episcleral venous pressure. MERCURY 1 and MERCURY 2 were Phase III studies that confirmed the superiority of Rocklatan to its individual components, netarsudil and latanoprost. The studies found that 82% of patients ultimately had an IOP ≤18mm Hg, 30.9% achieved at least a 40% reduction over baseline and 60% achieved a 30% reduction in IOP.
The side effect profile is similar to those of the component medications and includes conjunctival hyperemia (63%), instillation site pain (23%), conjunctival hemorrhage (13%) and verticillata (17.6%).9 With its broad mechanism of action, Rocklatan is a substantial enhancement to a well-established medication. It provides tremendous potential to supersede current prostaglandins and should receive strong consideration on all eligible patients when initiating therapy.
OHTS Update: Two Decades of DataEarly publications from the Ocular Hypertension Treatment Study (OHTS) showed that treatment of OHT (mean baseline IOP of ≥24.9mm Hg) was associated with a reduced risk of developing glaucoma (five-year risk of 4.4% when treated vs. 9.5% when observed). It showed that observed patients developed POAG an average of 2.7 years earlier than those who were treated. It also identified important factors to help accurately predict the risk of developing POAG, stratified into low, medium and high groups. Phase II discovered that for most patients delaying treatment in the observation group (treated after 7.5 years) only had a small amount of glaucomatous damage compared to the initial treatment group.52 Recently published in 2021, Phase III looked at 20-year follow-up data from OHTS, specifically incidence and severity of POAG in one or both eyes. After adjustment to exposure to treatment, the 20-year cumulative incidence of POAG in one or both eyes was 45.6% overall, 49.3% in the original observation (treated after 7.5 years) group and 41.9% in the original treatment group. The incidence of disease was greater in Black participants vs. other races (55.2% vs. 42.7%). Incidence breakdown by risk group was as follows: 31.7% in low risk, 47.6% in medium risk and 59.8% in the highest risk group. The 20-year incidence of visual field loss was 25.2%, with higher mean deviations for patients with bilateral optic disc deterioration and visual field loss compared with patients with unilateral optic disc deterioration and visual field loss. Although there was a low incidence of visual impairment, 11% of patients ended up with visual acuity worse than 20/40 and 1.2% of patients worse than 20/200. More intensive treatment was needed in 18.1% of OHTS participants, requiring at least one surgical procedure across the life of their management.53 Accordingly, the initial diagnosis of OHT does not always indicate a benign process will unfold. Another major finding of Phase III was that the likelihood of developing glaucoma within the 20-year study was roughly equivalent to the likelihood of death (total of 483 POAG cases and 515 deaths). This statistic, although morbid, further informs our prognostic conversations with patients.53 Ultimately, although Phase III of OHTS has added to our knowledge, it remains that clinicians need to evaluate all patient risks, including age, health status and personal preferences. A discussion with the patient is warranted. Inform them of their individual risks and develop a plan to meet their needs. Observation or postponing treatment, as long as there is vigilant monitoring of both structure and function, is reasonable, as is treatment when the potential burden of disease outweighs the burden of treatment.54 |
Setbacks
Although these agents are proven to work in clinical trials, there are many barriers to success with any new therapy. For one, drug novelty comes with a few setbacks, including lack of prescribing familiarity and high costs. As drugs enter the market, insurance coverage and reimbursement challenges may impact patient access to it. Manufacturer coupons and patient assistance programs can provide cost-lowering solutions depending on patient eligibility.18,19 Next, chronic use of topical ophthalmic drugs can lead to ocular surface disease due to toxicity and inflammation. This complex issue leads to discontinuation of drops, as patients do not typically suffer from discomfort or pain from glaucoma but do feel dry eye symptoms.
 |
|
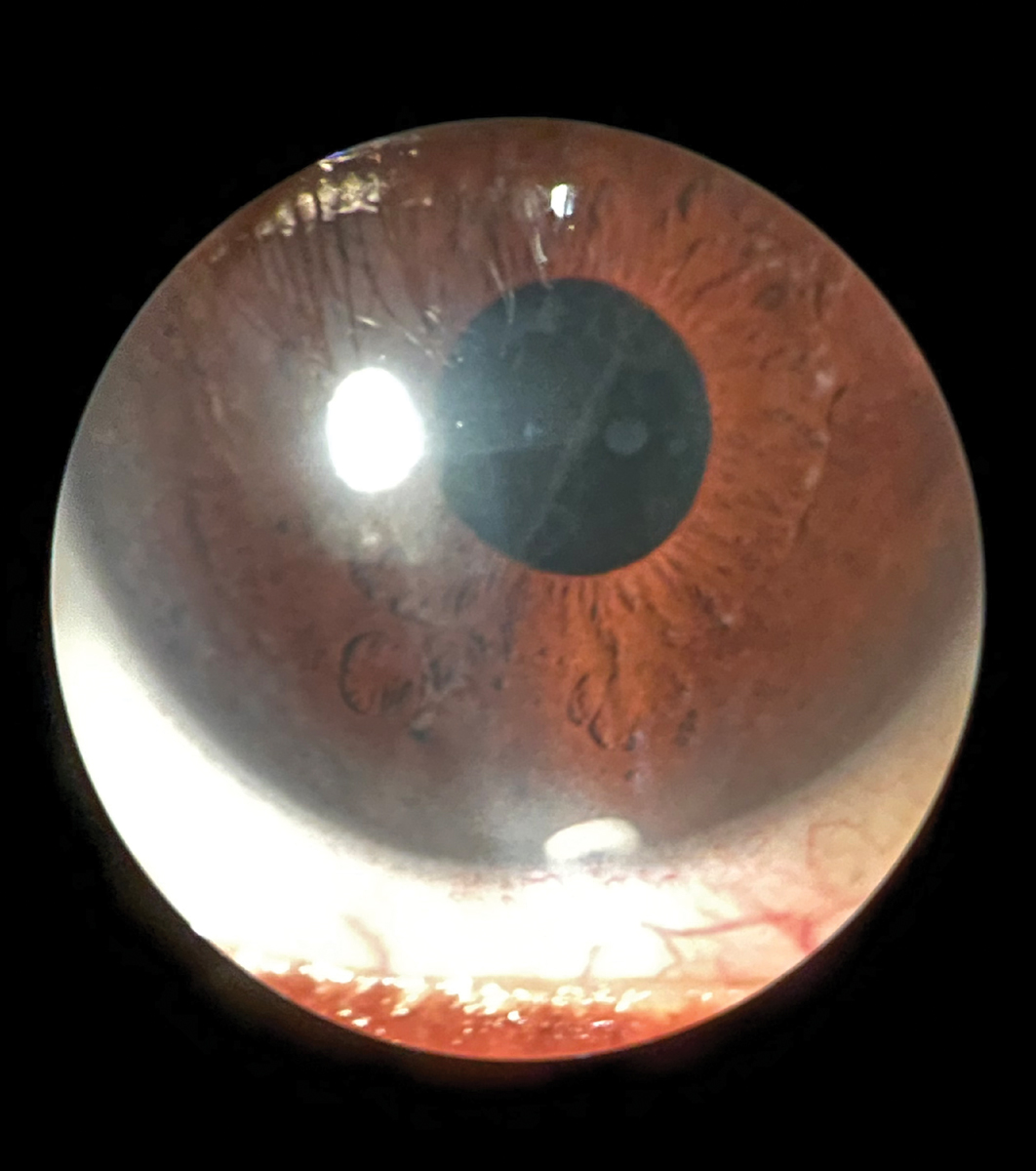
Anterior segment slit lamp photo of Durysta implant still visible at six-month follow-up. Click image to enlarge. |
The most widely used preservative, benzalkonium chloride, can have harmful effects especially over time.20,21 Alternative preservatives, available in brand-name drugs, seem to be less damaging to the ocular surface. Preservative-free options may show the least toxicity; however, the active ingredient can sometimes lead to ocular surface disease. Over-the-counter lubricants or prescription drops for ocular surface disease can be effective, but use of multiple drops in additional to glaucoma therapy can decrease patient adherence and be more costly.21
Medication adherence is one of the biggest barriers to success, with nonadherence rates being as high as 80%.22 In addition to bothersome side effects, other factors influencing nonadherence include forgetfulness, cognitive impairment, health illiteracy, affordability and incorrect administration.22,23
Knowledge of this reality, availability of newer drug delivery systems, and broader acceptance of first-line laser treatment offers a solution to lack of patient adherence, while contributing less damage to the ocular surface, leading to a more controlled, comfortable patient.21,24
Implants
One recently approved glaucoma therapy embraces intervention by direct intraocular delivery of a drug whose action is sustained for a substantial length of time, while subsequently decreasing the burden of adherence placed on the patient.
In March 2020, Durysta (bimatoprost intracameral implant, Allergan) became the first sustained-release intracameral implant to become FDA-approved for IOP lowering in patients with POAG and OHT.25 Durysta contains 10µg of bimatoprost within a rod-shaped, biodegradable, solid polymer drug delivery system that is 200µm in diameter and 1.1mm in length.
The delivery platform is the same as that used to deliver Ozurdex (dexamethasone, Allergan). The implant is supplied preloaded on a single-use, 28-gauge applicator designed to facilitate injection directly into the anterior chamber, typically settling in the inferior iridocorneal angle.
Once injected, the implant is designed to directly target the iris/ciliary body interface, acting to increase aqueous outflow through both the conventional and uveoscleral outflow pathways and provide slow and continuous release of bimatoprost over four to six months.25-27
Two Phase III 20-month studies (ARTEMIS 1 and ARTEMIS 2) showed that the mean IOP over 12 weeks after bimatoprost implantation is consistently lower than timolol maleate 0.5% BID when compared directly. In both ARTEMIS 1 and 2, implants were administered on day one, week 16 and week 32 of the study. IOP was then assessed at week 52 after the last implantation. Interestingly, the proportion of subjects requiring no additional treatment for a year after the third implant were 82.1% in ARTEMIS 1 and 77.5% in ARTEMIS 2. By comparison, in Phases I and II only 36% of subjects did not require additional treatment for a year after a single implant.
 |
|
In SLT, the laser straddles the TM. Click image to enlarge. |
It has been proposed that the extended duration of action after sequential implants may be explained by remodeling of the aqueous humor outflow pathway mediated by matrix metalloproteinase (MMP) ,which can lead to lower episcleral venous pressure.25,27,28 At present, Durysta is approved for one solitary administration. Although its long-term utility will depend on achieving an indication for repeated administration, the value of Durysta as an additional management tool is apparent.
Overall, sustained-release bimatoprost is safe, with a low incidence of serious adverse events in all three study phases. The most common of these were conjunctival hyperemia, eye irritation, foreign body sensation, conjunctival hemorrhage and eye pain, all of which were typically reported as secondary to the implantation procedure rather than the medication itself and abated within two weeks of the procedures.
The most common serious ocular adverse event was corneal endothelial cell loss. A ≥20% decrease in central endothelial cell density (CECD) was reported in 0% (ARTEMIS 1 and 2) of patients after the first administration of the 10ug implant, in 2.3% and 0.6% of patients after the second administration, in 4.1% and 3.5% after the third administration and in 10.2% and 8.1% of respective study eyes at month 20. Implants were removed in 3.6% and 2.9% of respective patients, most commonly for corneal edema or central endothelial cell loss.
Notably, patients in the ARTEMIS studies were required to have a CECD ≥1,800 cells/mm2 in order to exclude patients with significant corneal endothelial dysfunction.25,27,28 Ultimately, Durysta should not be used in patients with corneal endothelial dystrophy or those who have previously undergone corneal transplantation.
Although intracameral implantation is an excellent option, a 2016 study of glaucoma patients revealed that 55% preferred daily drop use over more aggressive treatments.29 A patient survey by the Glaucoma Service at Massachusetts Eye and Ear determining acceptance of six different drug delivery approaches showed an acceptance rate of 30% for an injectable anterior chamber implant. Contrarily, patients with more severe disease were more likely to consider alternatives to eye drops.30
Meanwhile, physicians may have a more positive outlook on sustained drug delivery, as a 2014 survey of ophthalmologists showed that 88.9% would consider using a newer drug delivery mechanism (i.e., a drug-eluting contact lens).31 However, these studies and surveys on patient and practitioner attitudes were done prior to FDA approval of any sustained delivery devices; thus, having tangible options in current practice may shift attitudes beyond what was previously theoretical.
Exam ToolsEvery examination should incorporate these assessments, which can provide more objective and quantitative information. Funduscopy • Examine superior-temporal and inferior-temporal rim tissue and RNFL. • Distinguish cupping greater than pallor and visible laminar reconfiguration. • Expect a large cup-to-disc ratio for large optic discs. Gonioscopy • Studies indicate gonioscopy is the least-performed clinical examination tool in glaucoma care.55 —Our take: gonioscopy differentiates glaucoma types (i.e., PG, PXG, NVG, ACG, traumatic) along with the anterior segment evaluation. IOP • Despite being the only modifiable risk factor, note that other pressures—such as episcleral venous pressure, ocular perfusion pressure and cerebrospinal fluid pressure—influence optic nerve health. • Recognize true diurnal/nocturnal IOP varies. • Consider multiple untreated readings. • Acquire corneal thickness and hysteresis. OCT • Review raw scan quality and retinal layer segmentation. • Differentiate among ischemic, compressive and anomalous causes. • View neuroretinal rim thickness, circumpapillary RNFL thickness and macular ganglion cell thickness, looking for glaucomatous patterns of loss. Visual field • The World Glaucoma Association recommends at least two reliable visual fields within six months and two more visual fields within 18 months to establish an accurate baseline.56 —Our take: personalize test strategy (24-2C, 24-2, 10-2) based on topographical analysis of tissue. —Our take: assist in staging of severity of glaucoma. —Our take: focus on expanding and/or deepening of defect density and confirm defects are repeatable. • Discern slow progression from large, drastic changes caused by other conditions. |
Laser Focus
Another procedure that looks beyond pharmacotherapy and eliminates risk of medication nonadherence is selective laser trabeculoplasty (SLT), which uses a 532nm Q-switched, frequency-doubled Nd:YAG laser to deliver three nanoseconds of short-pulse therapy to the pigmented TM to increase aqueous outflow.32 Histopathological studies have shown SLT’s selective photothermolysis causes less collateral damage than its predecessor, argon laser trabeculoplasty, where heat generated within the pigmented cells dissipates and damages the surrounding TM tissue.32,33 SLT also increases aqueous outflow via biological changes such as modulation of gene expression, cytokine secretion, matrix metalloproteinase induction and TM remodeling.32,34-36
SLT received FDA approval in 2001 and has become increasingly more common as adjunct and first-line therapy for reduction of IOP in POAG and OHT patients.37-40 Clinical technique relies on the physician’s gonioscopy abilities, identification of the pigmented TM and knowledge of appropriate laser spot size and energy level.
Many studies have evaluated degrees of treatment and energy settings with respect to IOP lowering, resulting in current practice standards of 50 to 100 shots applied over 180˚ to 360˚ with energy levels around 0.6mJ to 1.4mJ.37-40 Successful application of laser is confirmed with visual feedback of minor tissue reaction or small microbubbles after each laser shot.40
SLT does reduce IOP but its effect subsides over time. Average IOP reduction following SLT is around 21% to 29% at six months, 16% to 30% at 12 months, 7% to 28% at two years, 24% to 25% at three years, 23% to 29% at four years, 22% to 32% at five years and 22% at six years. Treatment success is widely accepted to be an IOP reduction of greater than 20% from baseline.
Reported success rates vary from 66% to 75% at six months and diminish to 11% to 31% at five years.40,41 The repeatability of SLT mitigates this diminished return; it is safe and effective to provide additional SLT treatment to previously treated TM, with retreatment providing similar IOP reduction as primary SLT.42,43
SLT is not indicated if TM cannot be visualized. Therefore, it is paramount that the referring or procedure-performing physician have sufficient gonioscopy skills to recognize SLT candidacy. SLT is not always successful in isolation, which emphasizes the need to individualize treatment for each patient, including custom IOP target ranges and thorough assessment for disease progression with funduscopy and ancillary testing.44,45
The general increase in the incorporation of SLT has driven the need to compare the procedure to topical therapies. Review of current literature shows that SLT is as effective as topical medication for IOP control, including prostaglandin analog monotherapy or different topical medications used in combination.29,38,40,46 Of note are the results of the Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial, which investigated the efficacy of SLT as a primary treatment compared with topical medications in treatment-naïve patients over 36 months.
Primary SLT patients were at their individualized IOP targets over more clinical visits during the 36-month period compared with patients treated with eye drops (93% vs. 91%). Furthermore, the primary SLT group also demonstrated less disease progression and fewer surgical interventions. Drop-free and disease-controlled primary SLT patients, after one or two SLT therapies, equaled 85% at 12 months, 79% at 24 months and 74% at 36 months.
Other study factors of the LiGHT Trial were health-related quality of life and cost-effectiveness. Primary SLT was found to be more cost-effective than topical therapy over 36 months, but the health-related quality of life was not significantly different between the two groups.45 Thus, SLT is considered a safe and effective alternative to topical therapy for POAG and OHT patients, while avoiding adverse effects associated with eye drops, which may improve treatment adherence and overall patient quality of life.40,44,45
Other benefits of SLT include delivery of treatment using a short, outpatient method with quick recovery and a good safety profile.40 There are limited adverse events related to SLT; the LiGHT Trial deemed all to be transient and self-limiting, with the most common adverse event being ocular discomfort immediately post-procedure (23% of patients).45 This data supports the belief that SLT is a flexible treatment option for a variety of patient care scenarios, especially when topical therapy is not best practice. Implementing SLT into the optometric practice will strengthen overall glaucoma care by increasing the opportunity for patients to receive prompt treatment while reducing ocular surface disease and patient non-adherence.21,24,47
Clinical Takeaways
Prior to initiating or changing any treatment, the diagnosis of glaucoma should be confirmed, as treatment is typically lifelong and affects quality of life. Unfortunately, misdiagnosis of glaucoma is quite common.48 Patients should not be treated based on IOP alone, especially if the risk of conversion from OHT to POAG is low, based on age or corneal thickness (see “An OHTS Update”).49 Confirmation of glaucoma requires sound clinical fundoscopy and interpretation of technology (see “Exam Tools”).50,51
Glaucoma management is continually evolving, with new drug trials and longevity studies showing effectiveness of treatment. Practitioners should avoid remaining stagnant in their treatment protocols and patient discussions.
Dr. Ragha is an assistant professor at Southern College of Optometry (SCO) and practices in the areas of primary care and ocular disease. She is a Fellow of the American Academy of Optometry. Dr. Hogan is a clinical instructor at SCO and practices in the areas of ocular disease and minor optometric procedures. She is a Fellow of the AAO. Dr. Rixon is an attending optometrist at the Memphis VA. He has achieved Glaucoma Diplomate status through the AAO and is a member of the Optometric Glaucoma Society. They have no financial disclosures.
1. MedWatch: the FDA safety information and adverse event reporting. FDA. www.fda.gov/medwatch. Accesssed January 7, 2022. 2. Weinreb RN, Liebmann JM, Martin KR, et al. Latanoprostene bunod 0.024% in subjects with open-angle glaucoma or ocular hypertension: pooled Phase III study findings. J Glaucoma. 2018;27(1):7-15. 3. Weinreb RN, Ong T, Sforzolini BS, et al. A randomized, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99(6):738-45. 4. Liu JHK, Slight JR, Vittitow JL, et al. Efficacy of latanoprostene bunod 0.024% compared with timolol 0.5% in lowering intraocular pressure over 24 hours. Am J Ophthalmol. 2016;169:249-57. 5. Araie M, Sforzolini BS, Vittitow J, Weinreb RN. Evaluation of the effect of latanoprostene bunod ophthalmic solution, 0.024% in lowering intraocular pressure over 24h in healthy Japanese subjects. Adv Ther. 2015;32(11):1128-39. 6. Kawase K, Vittitow JL, Weinreb RN, et al. Long-term safety and efficacy of latanoprostene bunod 0.024% in Japanese subjects with open-angle glaucoma or ocular hypertension: the JUPITER Study. Adv Ther. 2016;33(9):1612-27. 7. Serle JB, Katz LJ, McLaurin E, et al. Two Phase III clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116-127. 8. Yamamoto K, Maruyama K, Himori N, et al. The novel rho-kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest Ophthalmol Vis Sci. 2014;55(11):7126-36. 9. Al-Humimat G, Marashdeh I, Daradkeh D, Kooner K. Investigational rho kinase inhibitors for the treatment of glaucoma. J Exp Pharmacol. 2021;13:197-212. 10. Akagi T, Okamoto Y, Kameda T, et al. Short‐term effects of different types of anti‐ glaucoma eyedrop on the sclero‐conjunctival vasculature assessed using anterior segment OCTA in normal human eyes: A pilot study. Journal of Clinical Medicine. 2020;9(12):1-12. doi:10.3390/jcm9124016 11. Chihara E, Dimitrova G, Chihara T. Increase in the OCT angiographic peripapillary vessel density by ROCK inhibitor ripasudil instillation: a comparison with brimonidine. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1257-64. 12. Sit AJ, Gupta D, Kazemi A, et al. Netarsudil improves trabecular outflow facility in patients with primary open angle glaucoma or ocular hypertension: a Phase II study. Am J Ophthalmol. 2021;226:262-9. 13. Moshirfar M, Parker L, Birdsong OC. Use of rho-kinase Inhibitors in ophthalmology: a review of the literature. Med Hypothesis Discov Innov Ophthalmol. 2018;7(3):101-11. 14. Bacharach J, Dubiner HB, Levy B, et al. Double-masked, randomized, dose-response study of AR-13324 vs. latanoprost in patients with elevated intraocular pressure. Ophthalmology. 2015;122(2):302-7. 15. Gonzalez LE, Boylan PM. Netarsudil for the treatment of open-angle glaucoma and ocular hypertension: a literature review. Ann Pharmacother. 2021;55(8):1025-36. 16. Villegas NC, Lee WS. Effectiveness of netarsudil as an additional therapy for glaucoma in patients already on maximally tolerated medical therapy. Clin Ophthalmol. 2021;15:4367-72. 17. Singh IP, Fechtner RD, Myers JS, et al. Pooled efficacy and safety profile of netarsudil ophthalmic solution 0.02% in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2020;29(10):878-84. 18. Stuart A. Drug Update: Vyzulta and Rhopressa. EyeNet. September 2018. www.aao.org/eyenet/article/drug-update-vyzulta-and-rhopressa. Accessed January 30, 2022. 19. Vyzulta. GoodRx. www.goodrx.com/vyzulta. Accessed January 30, 2022. 20. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350-5. 21. Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45(1):11-8. 22. Newman-Casey PA, Blachley T, Lee PP, et al. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122(10):2010-21. 23. Kesav NP, Capitena Young CE, Ertel MK, et al. Sustained-release drug delivery systems for the treatment of glaucoma. Int J Ophthalmol. 2021;14(1):148-59. 24. Ahmed IIK. An introduction to IG. Glaucoma Today. 2020. glaucomatoday.com/articles/2020-jan-feb/an-introduction-to-ig. Accessed January 30, 2022. 25. Sirinek PE, Lin MM. Intracameral sustained release bimatoprost implants (Durysta). Semin Ophthalmol. September 29, 2021. [Epub ahead of print]. 26. Shirley M. Bimatoprost implant: first approval. Drugs Aging. 2020;37(6):457-62. 27. Medeiros FA, Walters TR, Kolko M, et al. Phase III, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627-41. 28. Bacharach J, Tatham A, Ferguson G, et al. Phase III, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017-33. 29. Kennedy JB, SooHoo JR, Kahook MY. Selective laser trabeculoplasty: an update. Asia Pac J Ophthalmol (Phila). 2016;5(1):63-9. 30. Wang BB, Lin MM, Nguyen T, Turalba AV. Patient attitudes toward novel glaucoma drug delivery approaches. Digit J Ophthalmol. 2018;24(2):16-23. 31. Taniguchi EV, Kalout P, Pasquale LR, et al. Clinicians’ perspectives on the use of drug-eluting contact lenses for the treatment of glaucoma. Ther Deliv. 2014;5(10):1077-83. 32. Kagan DB, Gorfinkel NS, Hutnik CM. Mechanisms of selective laser trabeculoplasty: a review. Clin Exp Ophthalmol. 2014;42(7):675-81. 33. Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology. 2001;108(4):773-9. 34. Izzotti A, Longobardi M, Cartiglia C, et al. Trabecular meshwork gene expression after selective laser trabeculoplasty. PLoS ONE. 2011;6(7):e20110. 35. Lee JY, Kagan DB, Roumeliotis G, et al. Secretion of matrix metalloproteinase-3 by co-cultured pigmented and non-pigmented human trabecular meshwork cells following selective laser trabeculoplasty. Clin Exp Ophthalmol. 2016;44(1):33-42. 36. Alvarado JA, Katz LJ, Trivedi S, Amde SS. Monocyte modulation of aqueous outflow and recruitment to the trabecular meshwork following selective laser trabeculoplasty. 2010;128(6):731-7. 37. Chen E, Golchin S, Blomdahl S. A comparison between 90º and 180º selective laser trabeculoplasty. J Glaucoma. 2004;13(1):62-5. 38. Nagar M, Ogunyomade A, O’Brart DPS, Howes F, Marshall J. A randomized, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89(11):1413-7. 39. Tang M, Fu Y, Fu MS, et al. The efficacy of low-energy selective laser trabeculoplasty. Ophthalmic Surg Lasers Imaging. 2011;42(1):59-63. 40. Garg A, Gazzard G. Selective laser trabeculoplasty: past, present and future. Eye (Lond). 2018;32(5):863-76. 41. Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clin Ophthalmol. 2015;9:833-41. 42. Ayala M. Intraocular pressure reduction after initial failure of selective laser trabeculoplasty (SLT). Graefes Arch Clin and Exp Ophthalmol. 2014;252(2):315-20. 43. Khouri AS, Lin J, Berezina TL, Maltzman B, Fechtner RD. Repeat selective laser trabeculoplasty can be effective in eyes with initial modest response. Middle East Afr J Ophthalmol. 2014;21(3):205-9. 44. Garg A, Nathwani N, Garway-Heath D, et al. Primary selective laser trabeculoplasty for open-angle glaucoma and ocular hypertension: clinical outcomes, predictors of success and safety from the laser in glaucoma and ocular hypertension trial. Ophthalmology. 2019;126(9):1238-48. 45. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty vs. eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicenter randomized controlled trial. Lancet. 2019;393(10180):1505-16. 46. Katz LJ, Steinmann WC, Kabir A, et al. Selective laser trabeculoplasty vs. medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460-8. 47. Jones L, Konstantakopoulou E, Gazzard G. Selective laser trabeculoplasty (SLT) performed by optometrists for patients with glaucoma and ocular hypertension: A scoping review. BMJ Open Ophthalmol. 2021;6(1):e000611. 48. Greenfield DS. Glaucomatous vs. nonglaucomatous optic disc cupping: clinical differentiation. Semin Ophthalmol. 1999;14(2):95-108. 49. Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20. 50. Sayed MS, Margolis M, Lee RK. Green disease in optical coherence tomography diagnosis of glaucoma. Curr Opin Ophthalmol. 2017;28(2):139-53. 51. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern. Ophthalmology. 2021;128(1):P71-P150. 52. Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol. 2010;128(3):276-87. 53. Kass MA, Heuer DK, Higginbotham EJ, et al. Assessment of cumulative incidence and severity of primary open-angle glaucoma among participants in the ocular hypertension treatment study after 20 years of follow-up. JAMA Ophthalmol. 2021;139(5):558-66. 54. Anderson DR. Clinical application of the 20-year results from the ocular hypertension treatment study. JAMA Ophthalmol. 2021;139(10):1146. 55. Stanley J, Huisingh CE, Swain TA, et al. Compliance with primary open-angle glaucoma and primary open-angle glaucoma suspect preferred practice patterns in a retail-based eye clinic. J Glaucoma. 2018;27(12):1068-72. 56. Weinreb R, Garway-Heath D, Leung C. Progression of Glaucoma. In: World Glaucoma Association. 8th ed. Kugler Publications; 2011. |

