In the fall of 2010, a 66-year-old white female presented for a comprehensive exam with complaints of decreased vision O.D. and O.S. at both distance and near. She reported that her last ophthalmic evaluation was approximately five years earlier. She had recently seen her primary care physician who noticed something she described as a cataract O.S., and he referred her to me.

Her current medications included Lipitor (atorvastatin, Pfizer), Micardis (telmisartan, Boehringer Ingelheim Pharmaceuticals), verapamil, 81mg aspirin, Lovaza (omega-3-acid ethyl esters, GlaxoSmithKline), allopurinol and Celexa (citalopram HBr, Forest Pharmaceuticals). She reported an allergy to “cyclines.”
Diagnostic Data
Entering visual acuity was 20/50 O.D. and 20/70- O.S. Best-corrected acuity through mixed astigmatic correction was 20/25-O.D. and 20/50 O.S. Pupils were slightly asymmetric with O.D. at 4mm and O.S. at 5mm in ambient light. (The anisocoria did not change in bright or dim illumination.) Both pupils were slightly oval and irregular in appearance. There was no afferent pupillary defect in either eye. Extraocular motilities were full in all positions of gaze.
Slit lamp examination of her anterior segments was remarkable for exfoliative material present in the anterior segment of both eyes. Both anterior lens capsules demonstrated pseudoexfoliative (PXE) material in a circular pattern adjacent to the papillary margin. PXE material was also seen on the pupillary ruff O.U., and there were mild transillumination defects close to the papillary ruff O.U.
Intraocular pressure measured 34mm Hg O.D. and 35mm Hg O.S. at 10:15 a.m. Van Herick estimations of anterior chamber angles were grade III open O.U. Pachymetry of the central corneas measured 568μm O.D. and 573μm O.S.
Through dilated pupils, her right crystalline lens was characterized by grade I nuclear sclerosis, with anterior and posterior cortical spokes encroaching on the visual axis. The left lens was characterized by grade II+ nuclear cataracts and cortical cataracts on the visual axis. The asymmetry in lens changes corresponded with the asymmetry in best-corrected visual acuities.
Close stereoscopic examination of the optic nerves demonstrated characteristic glaucomatous optic neuropathy, left eye greater than the right, with cup-to-disc ratios of 0.45 x 0.65 O.D. and 0.50 x 0.75 O.S. Both superior and inferior temporal retinal rims were thinner, with the left more advanced than the right. The neuroretinal rims of either eye did not adhere to the ISNT (inferior-superior-nasal-temporal) rule. Retinal vasculature was characterized by grade 2 hypertensive retinopathy O.U. and grade 3 arteriolarsclerotic retinopathy O.U. Both macular evaluations were characterized by fine RPE granulation, with no drusen or suspected submacular activity. Posterior vitreous detachment was present bilaterally. The peripheral retinas were unremarkable.
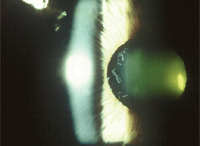
In this patient, PXE material is visible on the anterior lens capsule in a classic annular configuration. Remember:
pseudoexfoliation can affect many organs, including blood vessels and skin. Courtesy: Paul C. Ajamian, O.D.
I explained my findings to the patient was and requested her to return for formal visual field testing, optic nerve imaging and early morning IOP measurement.
When the patient returned two weeks later, her IOP was 36mm Hg O.D. and O.S. at 8:40 a.m. Threshold visual fields were unreliable, with high fixation losses and high false positives. Retinal tomographic imaging of both optic nerves corresponded with clinical slit lamp evaluations and demonstrated compromised neuroretinal rims, left eye greater than the right.
Gonioscopy demonstrated PXE material deposited in each angle, with the left showing more PXE material than the right. Sampaolesi’s line was noticeably present in both eyes.
Discussion
Initially, the patient presented with complaints of decreased vision attributable to her cataracts, which must be addressed in the near future, at least in the left eye
More pressing, though, is her uncontrolled glaucoma. So, I initially prescribed 1 drop Travatan Z (travoprost, Alcon), O.U. h.s. This resulted in an overall decrease in IOP of approximately 20% to 25% O.U. Post-treatment pressures averaged in the upper 20s O.D. and O.S. But, we had set initial target pressures between 15mm Hg and18mmHg O.U., and we were obviously not at target.
I added Alphagan P (brimonidine tartrate, Allergan) b.i.d. O.U., which resulted in an additional lowering of IOP to the mid 20s O.U.
Ultimately, on a regimen of 1 drop Travatan Z O.U. h.s. and 1 drop Combigan (brimonidine tartrate/timolol maleate, Allergan) O.U. b.i.d., her post-treatment IOP averaged 19mm Hg to 22mm Hg O.D. and O.S. Clearly still not at target, the patient is currently scheduled for bilateral selective laser trabeculoplasties.
This case is very typical of the difficulty in controlling IOP in patients with pseudoexfoliative glaucoma (PXG). The PXE material that is deposited throughout the anterior chamber obstructs the aqueous outflow, resulting in elevated IOP, often not reducible to target pressures with topical therapy alone.
But we need to keep in mind that this same PXE process has effects in the posterior chamber as well, primarily at the level of the capsular zonules. Regardless of the presence of glaucoma, patients with PXE have weakened zonular attachments that can complicate cataract surgery. Also complicating cataract surgery in these patients is the impact of PXE on the iris, as these patients often have inhibited dilating capabilities and a somewhat reduced integrity of the blood aqueous barrier. Cataract surgeons must be aware of the presence of PXE prior to surgery to take necessary intraoperative care to reduce zonular dehiscense.
While all patient populations can have PXE, it is more predominant in those of Scandanavian descent.1 Most estimates of its prevalence in the U.S. indicate that approximately 15% to 25% of all cases of open-angle glaucoma are of PXE origin.2 As eye care providers, we are well aware that patients with PXE have a higher incidence of converting to glaucoma from ocular hypertension, and that their glaucoma is often difficult to treat. When present in one eye, the likelihood of the fellow eye becoming involved is about 40%, and diurnal IOP variations in those with PXE or PXG are markedly higher than in other forms of glaucoma.3,4
We know that PXE is more than just glaucoma and requires more aggressive therapy and more caution during cataract surgery, we must also keep in mind that PXE involves more than just the eye.
PXE, with its most easily identifiable signs found in the eye, is nonetheless a systemic condition. Throughout the body, it is associated with abnormalities of the basement membrane of all epithelial cells. Its effects can be seen in the kidneys, skin, meninges, heart, lungs and blood vessels.
PXE appears to be maternally inherited, though the exact inheritance pattern, if any, is not clearly identified. In both the development of PXE and PXG, it appears to be related to LOXL1 gene expression.5 Furthermore, PXE has been associated with higher levels of homocysteine, which is elevated in patients with cardiovascular disease, and is believed to play a role in the development of peripheral vascular disease.6,7
While the exact relationships between PXE, PXG and systemic conditions with higher morbidity (such as vascular disease) have yet to be fully elucidated, I would not be surprised if in the near future those patients we identify clinically as having PXE or PXG will undergo a standard list of systemic evaluations to gauge those other, non-ocular risks.
As for the patient in this case, she is scheduled and awaiting bilateral SLT.
1. Ringvold A. Epidemiology of glaucoma in northern Europe. Eur J Ophthalmol. 1996 Jan-Mar;6(1):26-9.
2. Karger RA, Jeng SM, Johnson DH et al. Estimated incidence of pseudoexfoliation syndrome and pseudoexfoliation glaucoma in Olmsted County, Minnesota. J Glaucoma. 2003 Jun; 12(3): 193-7.
3. Altintas O, Yuksel N, Karabas VL, et al. Diurnal intraocular pressure variation in pseudoexfoliation syndrome. Eur J Ophthalmol. 2004 Nov-Dec;14(6):495-500.
4. Schlotzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006 May;141(5):921-937.
5. Khan TT, Li G, Navarro ID, et al. LOXL1 expression in lens capsule tissue specimens from individuals with pseudoexfoliation syndrome and glaucoma. Mol Vis. 2010 Nov 2;16:2236-41.
6. Leibovitch I, Kurtz S, Shemesh G, et al. Hyperhomocystinemia in pseudoexfoliation glaucoma. J Glaucoma. 2003 Feb;12(1):36-9.
7. Praveen MR, Shah SK, Vasavada AR, et al. Pseudoexfoliation as a risk factor for peripheral vascular disease: a case-control study. Eye (Lond). 2010 Dec 3. [Epub ahead of print]

