
Multiple sclerosis (MS) is the most common demyelinating disorder of the central nervous system. More than 350,000 people in the
Early diagnosis and treatment with disease-modifying therapies can delay the development of future clinical events and overall disease progression. So, we must stay current with new developments in the diagnosis and management of MS.
MS Subtypes
In 1996, the National Multiple Sclerosis Society standardized the definitions of the different MS subtypes:3
Relapsing-remitting MS (RRMS), which is characterized by clinical flare-ups (relapses), followed by extended periods of no clinically evident new disease activity (remission). This is the most common early clinical course after the initial demyelination event in most patients with MS.
Secondary-progressive MS (SPMS), which is characterized by progressive neurological dysfunction between clinical flare-ups and no clear remission periods. Many cases of RRMS progress to this pattern. SPMS causes the greatest amount of disability and is the most common form of MS seen clinically.
Primary-progressive MS (PPMS), which is characterized by continuously progressive neurological dysfunction, with no clear flare-ups. This form only occurs in about 10% of MS patients and more commonly affects patients older than 50.
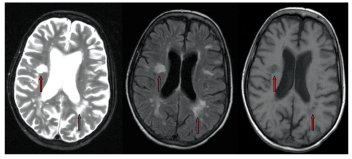
Left: MRI of MS lesions. Arrows point to demyelinating lesions. The axial T2-weighted, large lesion oriented perpendicular to the lateral ventricle is an example of
Ocular Findings
There are numerous neuro-ophthalmic findings in MS, ranging from subclinical to visually disabling. These include:
Optic neuritis. This is the most common ocular presentation. It is the initial clinical manifestation of MS in up to 20% of patients and occurs in more than 50% of patients over the course of the disease.4 While concurrent bilateral clinical optic neuritis presentations are rare, sequential involvement of the optic nerves is common.
Periorbital pain is common with most cases that present with the retrobulbar inflammation and a normal-appearing optic disc. However, about one-third of optic neuritis patients present with a mildly swollen optic disc (papillitis); hemorrhages or exudates are rarely associated with the papillitis.5 While visual function returns to better than 20/40 in most patients, low-contrast visual acuity or other visual quality factors may stay reducedeven 15 or more years after the initial optic neuritis event.6,7
MS Treatments Enter the Next Generation During acute flare-ups of MS symptoms, corticosteroids (oral prednisone and/or intravenous [IV] methylprednisolone), are the mainstay of treatment. These agents help shorten the duration of the attack and hasten recovery. Alemtuzumab, which is FDA approved for treatment of some forms of leukemia. It has shown a 70% reduction in the progression of RRMS, but the risk of autoimmune thyroiditis and idiopathic thrombocytopenia purpura may limit it as a first-line MS medication. Rituxmab, which has shown a significant reduction of gadolinium-enhancing lesions in RRMS patients and may also be effective against neuromyelitis optica, which involves demyelination of the optic nerves and spinal cord. Fingolimod, which is not a monoclonal antibody medication but instead works by preventing lymphocytes from entering the CNS during the inflammation stage of RRMS, has demonstrated a reduction in both relapses and gadolinium-enhancing lesions in RRMS, and has the advantage of being an oral medication vs. the injection route of the other medications.
Ocular motility dysfunction. Inaccurate saccadic intrusions and nystagmus, particularly internuclear ophthalmoplegia (INO), are frequent clinical manifestations of MS. With INO, there is incomplete or slowed adduction along with an abducting nystagmus. INO is considered a hallmark of MS when present unilaterally in young patients, or bilaterally in patients of any age.8
However, corticosteroids do not improve the final degree of recovery or significantly alter the long-term course of the disease. The Optic Neuritis Treatment Trial (ONTT) has shown that among patients initially treated with IV corticosteroids, more than 50% of those patients have converted to clinically definite MS after 15 years.6,7 While oral corticosteroids are widely used as monotherapy for many systemic MS flare-ups, they should not be used alone for treatment of optic neuritis because of the potential for new attacks.34
The primary disease-modifying therapies currently used in the
While there is a difference in the number of MRI lesions seen over time with the different therapies, interferons and glatiramer acetate generally reduce the amount of relapses by about one third.35-38 The major differences in the medications are the mechanism of action, the modes of delivery (intramuscular vs. subcutaneous), frequency of dosing (daily, every other day, weekly) and side effects. Regardless of which disease-modifying treatment the patient is placed on, treatment should be started early in the course of the disease.39
Two additional medications are available, but are limited in use due to a strong association with potentially fatal complications. Mitoxantrone, a chemotherapeutic agent used to treat many cancers, is sometimes employed for secondary-progressive MS treatment. Its effectiveness is questionable based on documented lack of decreased MRI lesions over time. Also, prolonged treatment can result in significant cardiotoxicity, so its use is further limited.40,41
Tysabri (natalizumab, Biogen IDEC), a monoclonal antibody, was pulled from the market in 2005, just months after FDA approval, because it was linked to three deaths from rarely seen progressive multifocal leukoencephalopathy (PML).42 Tysabri is available once again as a monotherapy for relapsing-remitting forms of MS (RRMS), with patients registered and carefully followed in risk-minimization programs. In August 2008, two new cases of PML were reported in
Besides Tysabri, several other monoclonal antibody medications are in clinical trials.43 These include:
Additional nystagmus presentations. These include upbeat, downbeat, gaze-evoked, pendular, vestibular and periodic alternating.
Cranial nerve palsies. While not as common as impaired saccades or nystagmus, isolated cranial nerve palsies involving the third, fourth or sixth nerve can be the initial clinical manifestation of MS.9
Uveitis. The incidence of uveitis in MS patients is about 1%; but, that is 10 times higher than the incidence in the general population.10,11
The clinical presentation is usually bilateral. Pars planitis and panuveitis are the most common forms. While much less frequent, isolated anterior uveitis in either the granulomatous or nongranulomatous form can also occur.
Retinal periphlebitis. This presents as a combination of venous sheathing with exudation and hemorrhaging.12 Periphlebitis is more common during the active stage of MS and can indicate progression of neurologic dysfunction.13
Magnetic Resonance Imaging
The diagnostic work-up for MS may include analysis of cerebrospinal fluid, looking for oligoclonal bands as a sign of elevated immunoglobulin G (IgG), and serologic testing to check vitamin B12 levels or rule out other disorders.
However, MRI is the test of choice to help establish the diagnosis. MRI studies that demonstrate lesions consistent with demyelination help establish a diagnosis of MS, predict disease progression and monitor treatment results. Lesions secondary to MS are found primarily in the brain white matter and spinal cord, but they can also involve the gray matter. Typically, the lesions are ovoidal in shape and are oriented perpendicular to the lateral ventricles in the periventricular area, sometimes referred to as
Different MRI imaging techniques can provide information on the age of the lesions, disease progression and amount of overall brain atrophy. Techniques include:
T2-weighted scanning, which is very sensitive to any edema that occurs with brain tissue changes. It is the single best type of image to look for MS lesions, which appear as bright hyperintense areas.
T2-weighted FLAIR images. FLAIR (fluid-attenuated inversion recovery) is a technique that reduces the signal of free fluid found in the cerebrospinal fluid of the ventricles during T2-weighted scanning. This allows small tissue abnormalities to be highlighted against the darker ventricles.
T1-weighted scanning with gadolinium contrast. While T2-weighted and T2-weighted FLAIR images are the best for detecting the demyelinating lesions of MS, they have one major disadvantage: Active and older inactive lesions both image similarly. T1-weighted scanning with gadolinium contrast, however, lets you determine which lesions are relatively new.
Fresh MS lesions cause an inflammatory breakdown of the blood-brain barrier that allows the gadolinium to leak into and enhance the area of the lesion. However, this enhancement will cease after the lesion is two to four weeks old.14
T1-weighted scanning without contrast. T1-weighted MS lesions, when not using contrast, are either isointense (appears grayish, similar to the surrounding tissue) or hypointense (darker than the surrounding tissue). The hypointense T1- weighted lesions, sometimes called black holes, represent permanent tissue loss and axonal destruction. They are strong predictors of whole-brain atrophy leading to future physical disability.15
Optical Coherence Tomography
The use of OCT in the diagnosis and management of MS is still being developed.
The retinal nerve fiber layer (RNFL) consists of unmyelinated axons that arise from the ganglion cell layer, making it a unique area from which we can directly measure the loss of axons from MS without the measurements being complicated by the loss of myelin mass. Many studies have found thinning of the RNFL in patients with MS, including patients with a history of acute optic neuritis and patients that lack any history of optic neuritis.16-22 The sector that appears to have the greatest RNFL loss is the temporal quadrant, which is consistent with the histological site of RNFL atrophy in MS.20,22-24 Studies have shown a correlation between RNFL loss on OCT and decreased visual acuity, decreased contrast sensitivity and mean visual field deviation.21,23
Different OCT studies show that the severity of an acute optic neuritis event has a large bearing on the amount of axonal loss. In several studies, even asymptomatic patients without a history of optic neuritis show reduced RNFL thickness, which suggests that a subclinical optic nerve degeneration occurs in MS.22,23 Because patients with MS may develop other disorders that compromise the optic nerve, consider doing an OCT to document possible subclinical nerve damage secondary to MS, and as a baseline to refer to in the future.
One question remains: Are changes in the retinal axons similar to changes in the brains of patients with MS? If so, OCT potentially may be used as a biomarker for both retinal damage and damage to the central nervous system secondary to MS.
And, OCT may be able to augment MRI and similar studies of MS-related disease activity. Research has shown that RNFL loss shows different patterns based on MS subtypes that occur more frequently in the progressive subtypes of MS vs. the relapsing-remitting subtype, even when adjusted for duration of disease.21,23
Can Vitamin D Help Prevent MS? Increased vitamin D levels are associated with a lower risk of developing MS.44,45 Indeed, the incidence of MS increases with further distance from the equator.46 One theory: Increased sunlight exposure at the lower latitudes closer to the equator results in increased vitamin D levels, which may have a protective effect.47 Additionally, MRI studies have shown that when vitamin D levels are low, the number of active lesions increases in MS patients.48,49
Vitamin D, an immunomodulator, is directly involved with regulatory/suppressor T cells, which are usually suppressed in MS patients.50,51 The most effective dose of vitamin D is unknown. One study that evaluated high-dose vitamin D in MS patients found no significant adverse effects and showed a decrease in the number of MRI lesions over time.52
Visual Evoked Potentials
VEPs usually are not indicated in a patient who presents with a classic acute optic neuritis. However, patients sometimes present with a low-grade slowly progressive, or subclinical, form of optic neuritis in which VEPs can help detect optic nerve compromise.
VEPs can help establish an otherwise questionable MS diagnosis. Several studies have shown that MS patients who have baseline abnormalities on MRI studies and abnormal VEPs have a 2.5 to 9 times higher risk of progressing to clinically definite MS than patients with normal VEPs.25-27
With demyelination, the conduction speed of the nerve fiber slows down, causing both a delay and amplitude attenuation of the P100 wave. The P100 wave of the pattern VEP is the most common marker used when looking at MS cases because it is a very consistent wave with a latency around 100 milliseconds in normal subjects. While the attenuation of the P100 wave corresponds more precisely to loss of axons, there is a large variability between patients. So, it is more common to look at the increased latency in determining if the nerve has been damaged.
Even among MS patients without a history of optic neuritis, as many as 90% can have delayed-pattern VEPs.28 However, while VEPs can remain abnormal for a year or longer after an optic neuritis event, the P100 can recover to normal in some patients.29,30 Also, the upper visual field contributes less to the total VEP signal than the lower field, so an optic neuritis patient with mostly superior field loss may not show a significant P100 delay.31
A newer type of VEP, the multifocal VEP (mfVEP), plots out what the electrical response of the visual pathway would be for specific areas of the visual field using mathematical modeling. These areas of abnormal waveforms often correspond to the visual field defects in the MS patient and are thought to represent local areas of demyelination in the optic nerve.32 The mfVEP is significantly more sensitive at detecting abnormalities of the optic nerve in MS patients who may have a normal pattern VEP, such as a patient with primary upper field loss. As with the standard VEP, newly diagnosed MS suspects with abnormal mfERGs are more likely to progress to clinically definite MS.33
Visual dysfunction plays a prominent role in the clinical presentation of MS, so O.D.s are often the ones to make the initial diagnosis. So, we must keep abreast of new technologies and therapies that may come into play when managing patients with MS.
Dr. Woods is an associate professor at Nova Southeastern University College of Optometry. He is Director of Electrodiagnostic Service and teaches courses on neuro-ophthalmic disease, ocular manifestations of systemic disease, and clinical medicine/physical diagnosis.
1. Noonan CW, Kathman SJ, White MC. Prevalence estimates for MS in the
2. Mowry EM, Balcer LJ, Galetta SL. Multiple sclerosis and the ophthalmologist. Comp Ophthalmol Update 2007 Jan-Feb:8(1):39-49.
3. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (
4. Jacobs DA, Galetta SL. Multiple sclerosis and the visual system. Ophthalmol Clin North Am 2004 Sep;17(3):265-73.
5. Nilsson P, Larsson EM, Maly-Sundgren P, et al. Predicting the outcome of optic neuritis: evaluation of risk factors after 30 years of follow-up. J Neurol 2005 Apr;252(4):396-402.
6. Bhatti MT for the Optic Neuritis Study Group. The final 15-year follow-up report on the neurological outcome of the Optic Neuritis Treatment Trial. Presentation at the 34th Annual North American Neuro-Ophthalmology Society (NANOS) Meeting, March 8-13, 2008;
7. Keltner J, Johnson C, Cello K, et al. A 15-year summary of abnormal visual fields in the Optic Neuritis Treatment Trial (ONTT). Presentation at the 34th Annual North American Neuro-Ophthalmology Society (NANOS) Meeting, March 8-13, 2008;
8. Reynolds SA, Woods AD, Pizzimenti JJ. Bilateral internuclear ophthalmoplegia as the presenting sign of multiple sclerosis: an interdisciplinary approach to diagnosis and management. The Internet Journal of Allied Health Sciences and Practice. Available at: http://ijahsp.nova.edu/articles/ Vol2number3/Bilateral_Internuclear_Ophthalmoplegia-Reynold.htm (Accessed June 24, 2008).
9. Thmke F, Lensch E, Ringel K, Hopf HC. Isolated cranial nerve palsies in multiple sclerosis. J Neurol Neurosurg Psychiatry 1997 Nov;63(5):682-5.
10. Smith JR, Rosenbaum JT. Neurological concomitants of uveitis. Br J Ophthalmol 2004 Dec;88(12):1498-9.
11. Biousse V, Trichet C, Bloch-Michel E, Roullet E. Multiple sclerosis associated with uveitis in two large clinic-based series. Neurology 1999 Jan;52(1):179-81.
12. Engell T,
13. Tola MR, Granieri E, Casetta I, et al. Retinal periphlebitis in multiple sclerosis: a marker of disease activity. Eur Neurol 1993;33(2):93-6.
14. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001 July;50(1):121-7.
15. Paolillo A, Pozzilli C, Gasperini C, et al. Brain atrophy in relapsing-remitting multiple sclerosis: relationship with "black holes," disease duration and clinical disability. J Neurol Sci 2000 Mar 15;174 (2):85-91.
16. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 1999 Oct;40(11):2520-7.
17. Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 2005 Sep;58(3):383-91.
18. Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006 Feb;113(2):324-32.
19. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 2006 Jun;59(6):963-9.
20. Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, et al. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 2007 May;68(18):1488-94.
21. Pulicken M, Gordon-Lipkin E, Balcer LJ, et al. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 2007;69(22):2085-92.
22. Gundogan FC, Demirkaya S, Sobaci G. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis? A structural and functional evaluation. Invest Ophthalmol Vis Sci 2007 Dec;48(12):5773-81.
23. Henderson AP, Trip SA, Schlottmann PG, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 2008 Jan;131(Pt 1):277-87.
24. Kerrison JB, Flynn T, Green WR. Retinal pathologic changes in multiple sclerosis. Retina 1994;14(5):445-51.
25. Matthews WB, Wattam-Bell JR, Pountey E. Evoked potentials in the diagnosis of multiple sclerosis: a follow-up study. J Neurol Neurosurg Psychiatry 1982 Apr;45(4):303-7.
26. Hume
27. Lee KH, Hashimoto SA, Hooge JP, et al. Magnetic resonance imaging of the head in the diagnosis of multiple sclerosis: a prospective 2-year follow-up with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology 1991 May;41(5):657-60.
28. Halliday AM,
29. Frederiksen JL, Petrera J. Serial visual evoked potentials in 90 untreated patients with acute optic neuritis. Surv Ophthalmol 1999 Oct;44 Suppl 1:S54-62.
30. Asselman P, Chadwick DW, Marsden DC. Visual evoked responses in the diagnosis and management of patients suspected of multiple sclerosis. Brain 1975 Jun;98(2):261-82.
31. Fortune B, Hood DC. Conventional pattern-reversal VEPs are not equivalent to summed multifocal VEPs. Invest Ophthalmol Vis Sci 2003 Mar;44(3):1364-75.
32. Hood DC, Odel JG, Zhang X. Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci 2000 Nov; 41(12):4032-8.
33. Fraser C, Klistorner A, Graham S, et al. Multifocal visual evoked potential latency analysis: predicting progression to multiple sclerosis. Arch Neurol 2006 Jun;63(6):847-50.
34. Beck RW, Cleary PA. Optic Neuritis Treatment Trial. One-year follow-up results. Arch Ophthalmol 1993 Jun;111(6):773-5.
35. PRISMS Study Group and the University of British
36. Paty DW, Li D; UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. 1993[classical article]. Neurology 2001 Dec;57(12 Suppl 5)S10-5.
37. Simon JH, Jacobs LD, Campion M, et al. Magnetic resonance studies of intramuscular interferon beta-1a for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group. Ann Neurol 1998 Jan;43(1):79-87.
38. Li DK, Paty DW. Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing-remitting multiple sclerosis. Prevention of Relapses and Disability by Interferon-beta1a Subcutaneously in Multiple Sclerosis. Ann Neurol 1999 Aug;46(2):197-206.
39. Coyle PK, Hartung HP. Use of interferon beta in multiple sclerosis: rationale for early treatment and evidence for dose- and frequency-dependent effects on clinical response. Mult Scler 2002 Feb;8(1):2-9.
40. Hartung HP, Gonsette R, Knig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002 Dec 21-28;360(9350): 2018-25.
41. Goodin DS, Arnason BG,
42.
43. DeAngelis T, Lublin F. Multiple sclerosis: new treatment trials and emerging therapeutic targets. Curr Opin Neurol 2008 June; 21(3): 261-71.
44. Munger KL, Zhang SM, O"Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004 Jan 13;62(1):60-5.
45. Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006 Dec; 296(23):2832-8.
46. Kurtzke JF. Geography in multiple sclerosis. J Neurol 1977 Apr 28; 215(1):1-26.
47. Goldberg P. Multiple sclerosis: vitamin D and calcium as environmental determinants of prevalence (a viewpoint). Part 1: sunlight, dietary factors and epidemiology. Int J Environ Studies 1974; 6:19-27.
48. Auer DP, Schumann EM, Kmpfel T, et al. Seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 2000 Feb;47(2):276-7.
49. Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 2000 Aug;48(2):271-2.
50. Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand) 2003 Mar;49(2):277-300.
51. Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 2004 Apr 5;199(7):971-9.
52. Kimball SM, Ursell MR, O"Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr 2007 Sep;86(3):645-51.

