 |
|
|
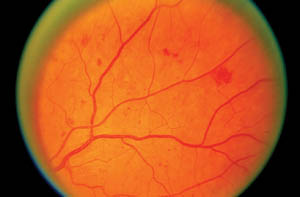
Telemedicine readers use ETDRS standard photographs, such as
this one showing HMAs, as a basis for comparison. A patient who
has equal or lesser amounts of HMAs in any retinal quadrant,
with
no
other diabetic retinopathy, would be diagnosed with mild non-proliferative diabetic retinopathy. However, a patient with four
retinal quadrants greater than this would be diagnosed with severe
non-proliferative diabetic retinopathy.
|
The pandemic of diabetes is on the rise in the United States and across the globe, resulting in growing numbers of new-onset blindness in working-age adults. The National Eye Institute reports that nearly half (40% to 45%) of Americans with diabetes have diabetic retinopathy. The NEI estimates that 7.7 million Americans ages 40 and older have some degree of diabetic eye disease. That represents an 89% increase since 2000, and projections anticipate another jump of 75%, to 13.5 million, by 2020.
In short, we can expect a huge influx of diabetes patients requiring eye care over the next few years. But how can we handle all of them?
Standardized diagnostic protocols will help. So can telemedicine. I’ve seen evidence of this with my own eyes. For the past seven years, I’ve been fortunate to be part of the Indian Health Service-Joslin Vision Network (IHS-JVN) Diabetic Retinopathy Telemedicine Program. My experience with this innovative technology has shown me that it’s also relevant in conventional clinic-based eye care.
This article offers some clinical insights for better diagnosing and managing diabetic eye disease, which I’ve learned through my experiences reading the images of more than 20,000 patients from across the country.
Have Some Standards
 | |
|
One of the retina telemedicine reading stations at the IHS-JVN National Reading Center in Phoenix. The glasses are used to view stereoscopic images.
|
Most of us were trained to diagnose diabetic retinopathy (DR) by clinical impression; however, the science of DR diagnosis was developed in a standardized fashion. Many of the features of the IHS-JVN standardized approach to diagnosis and management of DR can be easily implemented in a typical eye clinic.
While the scope of this article doesn’t allow a detailed discussion of the evidence basis for risk stratification of DR, we can easily refer to the Early Treatment Diabetic Retinopathy Study (ETDRS) and its standardized photographs to assist in standards-based clinical diagnosis of DR.1
The key is the quantification/localization of critical features, such as hemorrhages/microaneurysms (HMA), exudates, intraretinal microvascular anomalies (IRMA), venous caliber changes (as seen in venous beading) and neovascularization. A few of the ETDRS standard photographs, shown here, provide examples of these features and how they can be used for comparison to support your clinical diagnosis.
Although sophisticated telemedicine technology—using computer image enhancement and computer-assisted decision support—provides considerable diagnostic advantages for IHS-JVN readers, reliable and reproducible clinical diagnosis can be achieved without these digital enhancements. Of course, it takes careful study and repeated use of these slides to become proficient in standards-based diagnosis of DR, but improving these skills improves diagnostic accuracy and consistency, and enhances patient management and referrals.
Here are some observations and lessons
I’ve learned from my IHS-JVN telemedicine experience that you can use in
conventional clinical practice.
Lesson 1: Small Pupils Can Cause Big Problems
In both nonmydriatic telecapture and mydriatic live-eye exam, small
pupil size can cause difficulty for clinicians. Because patients with
diabetes often dilate poorly, at slower rates and often have media
opacities, be certain to allow adequate time for patients to dilate.
This simple pearl is often forgotten in busy practice.

|
|
|
|
Cotton-wool spots (soft exudates).
This finding elevates the level of diabetic retinopathy to at least
moderate nonproliferative diabetic retinopathy.
|
|
|
 |
||
| This photo shows hard exudates in the macula, which can be used to substantiate the diagnosis of clinically significant macular edema. | ||
Lesson 2: Hone in on High-Risk Areas
Certain retinal locations, such as the posterior pole, the area immediately nasal to the optic disc and the areas superior and superior temporal to the optic disc—are more likely to demonstrate high-risk characteristics of DR. The JVN validation studies document the value of special scrutiny of these locations.2Although diabetic retinopathy certainly exists outside of these defined parameters, it’s unusual that the level of pathology in other locations would be greater than those within these three areas. This can be clinically useful for patients in which viewing the fundus proves difficult, such as those with media opacity, light sensitivity or poor fixation.
Lesson 3: Know the Risks
A careful review of systems can quickly reveal important data to help better define the patient’s risk of onset and progression of diabetic retinopathy:
Age of onset and duration of diabetes mellitus (DM)
Control of hemoglobin A1C, blood pressure and lipids
Presence of peripheral neuropathy
Renal failure
Amputation(s)
For example, knowing the patient’s duration of diabetes, HbA1C and other health conditions, such as hypertension, is of utmost importance. Specifically, the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) found that the prevalence of diabetic retinopathy varied from 28.8% in adults (age 30 and older) who had diabetes for fewer than five years to 77.8% in adults who had diabetes for 15 or more years.4
Clinically, I’ve observed that the duration of diabetes seems to be the greatest single risk factor for the development of diabetic retinopathy, particularly if the patient has poor glycemic control. Patients with other comorbidities, such as cardiovascular disease, will have earlier demonstration and progression of diabetic retinopathy and other vascular changes, so they may require more frequent follow-up.
 |
| This photo illustrates neovascularization of the disc (NVD). A patient who shows a comparable level of disc neovascularization is considered to have proliferative diabetic retinopathy with high-risk characteristics. |
Coexisting hypertensive retinopathy can often confound the diagnosis of diabetic retinopathy, but some clinical features help distinguish the predominant factor driving the clinical presentation. Flame-shaped retinal hemorrhages are suggestive of hypertensive retinopathy, but the cotton-wool spots that encircle the optic disc can also represent renal/nephropathy-associated changes. Cotton-wool spots that have a homogenous distribution throughout the posterior pole are most likely of diabetic origin.
Capillary nonperfusion of the retina, or “featureless retina,” is another subtle finding that assists in risk stratification of the diabetic retina, yet remains a point of controversy in the literature. In addition to capillary dropout, no overlying hemorrhages or cotton-wool spots can be observed in a retina that is otherwise pathologic for diabetic retinopathy. This appearance can represent an underlying ischemic zone, which increases the risk for neovascular progression.
Handle these cases with caution and be vigilant to rule out neovascular disease. These patients should have a more careful biomicroscopic evaluation of suspicious areas in question, using a contact fundus lens to rule out subtle IRMA and neovascularization elsewhere (NVE).
Depending on the degree of presumed capillary dropout, consider fluorescein angiography to better quantify the degree of nonperfusion, rule out macular ischemia (especially in such patients who also have reduced best acuity), as well as delineate areas suspicious for neovascular disease.
 |
|
Flame-shaped hemorrhages and
cotton-wool spots, characteristic of hypertensive or
renal/nephropathy-associated changes, encircle the optical disc 360
degrees.
|
 |
|
The oval shows a subtle area of
"featureless retina," a focal area of capillary dropout, at risk for
proliferative and pre-proliferative diabetic changes.
|
One of the advantages of the telemedicine program is the ability to use the patients’ fundus photographs as the platform for educating them about DR. Some diabetes patients have a hard time understanding how they are being affected by their condition, but a picture—particularly of their own fundus—provides compelling, intuitive instruction.
If you’re unable to take a photo of the patient’s own fundus, another photo showing DR can be used instead.
Take time to explain your diagnosis, recommendations and follow-up plan thoroughly. Make appropriate referrals to primary care practitioners for optimal glycemic, BP and lipid management. Building contacts with primary care physicians will also increase patient recruitment for diabetic eye exams.
Nearly 27% of Americans 65 or older have diabetes, the American Diabetes Association reports. If current trends continue, perhaps as many as one in every three Americans will have diabetes by 2050, according to the Centers for Disease Control and Prevention.
Improving the quality and standardization of the diabetic retinal exam will help to build your practice and better serve this growing and already underserved population.
Even so, long-standing trends suggest that the conventional eye clinic-based exams may not be effective in reaching all the patients with diabetes. Telemedicine in a primary care medical setting has shown the ability to help.
Be aware that telemedicine is not in competition with clinical optometry, because it often reaches a different sector of patients who are not receiving eye care. In fact, the future may hold a place for using telemedicine in small optometric practices in much the same way the IHS-JVN has, by coordinating care with primary care providers through electronic medical record referrals. Telemedicine has great potential to expand the boundaries of evidence-based DM eye care and to close established barriers to care.
Our ultimate goal is the same as yours: to provide the best available care we can to help diagnose and manage these patients.

In addition to being a certified telemedicine reader at the
IHS-JVN National Reading Center in Phoenix, Dr. Giles is a clinical
optometrist practicing at the Phoenix Indian Medical Center.
1. Early Treatment Diabetic Retinopathy Study Research Group. Fundus
photographic risk factors for progression of diabetic retinopathy.
ETDRS report number 12. Ophthalmology. 1991 May;98(5 Suppl):823-33.
2. Bursell SE, Cavallerano JD, Cavallerano AA, et al; Joslin Vision
Network Research Team. Stereo nonmydriatic digital-video color retinal
imaging compared with Early Treatment Diabetic Retinopathy Study seven
standard field 35-mm stereo color photos for determining level of
diabetic retinopathy. Ophthalmology. 2001 Mar;108(3):572-85.
3. Chow SP, Aiello LM, Cavallerano JD, et al. Comparison of
nonmydriatic digital retinal imaging versus dilated ophthalmic
examination for nondiabetic eye disease in persons with diabetes.
Ophthalmology. 2006 May;113(5):833-40.
4. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic
study of diabetic retinopathy. III. Prevalence and risk of diabetic
retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol.
1984 Apr;102(4):527-32.
5. Wilson C, Horton M, Cavallerano J, Aiello LM. Addition of primary
care-based retinal imaging technology to an existing eye care
professional referral program increased the rate of surveillance and
treatment of diabetic retinopathy. Diabetes Care. 2005 Feb;28(2):318-22.
6. Whited JD, Datta SK, Aiello LM, et al. A modeled economic
analysis of a digital tele-ophthalmology system as used by three federal
health care agencies for detecting proliferative diabetic retinopathy.
Telemed J E Health. 2005 Dec;11(6):641-51.

