In April 2016, optometry lost a giant when the author of the seminal work Primary Care of the Posterior Segment, Larry Alexander, OD, died. In addition to being an optometric physician, author and educator at the University of Alabama Birmingham School of Optometry, Dr. Alexander was a past president of the Optometric Retina Society (ORS). That group chose to honor his legacy by accepting case reports from optometric residents across the country relating to vitreoretinal disease.
This case, selected by the ORS Awards Committee, was co-winner of the sixth annual Larry Alexander Resident Case Report Contest. The contest is sponsored by Topcon, Visionix (Optovue) and Heidelberg.
Pathologic myopia (PM) represents a spectrum of degenerative ocular structural complications that arise secondary to high myopia. Excessive axial length elongation drives biomechanical stretching of the posterior globe often associated with retinal and choroidal changes that inevitably lead to severe and irreversible visual impairment. The prognosis of PM varies depending on the clinical features; thus, it is critical to identify and monitor high risk patients, appreciate the severity, and take a collaborative approach pursuing interventions for vision threatening sequelae. Moreover, identifying myopia at an early age and employing interventions to suppress progression to PM should become a top priority. This case report outlines the various manifestations and management options of PM.
This case report will discuss a female patient with pathologic myopia and outlines the various manifestations and management options.
Case Presentation
A 58-year-old Native American female presented for a routine eye examination with complaints of floaters and glare out of both eyes that she had experienced consistently for many years. She had yellow filter glasses that provided relief. She denied any photopsia or vision loss.
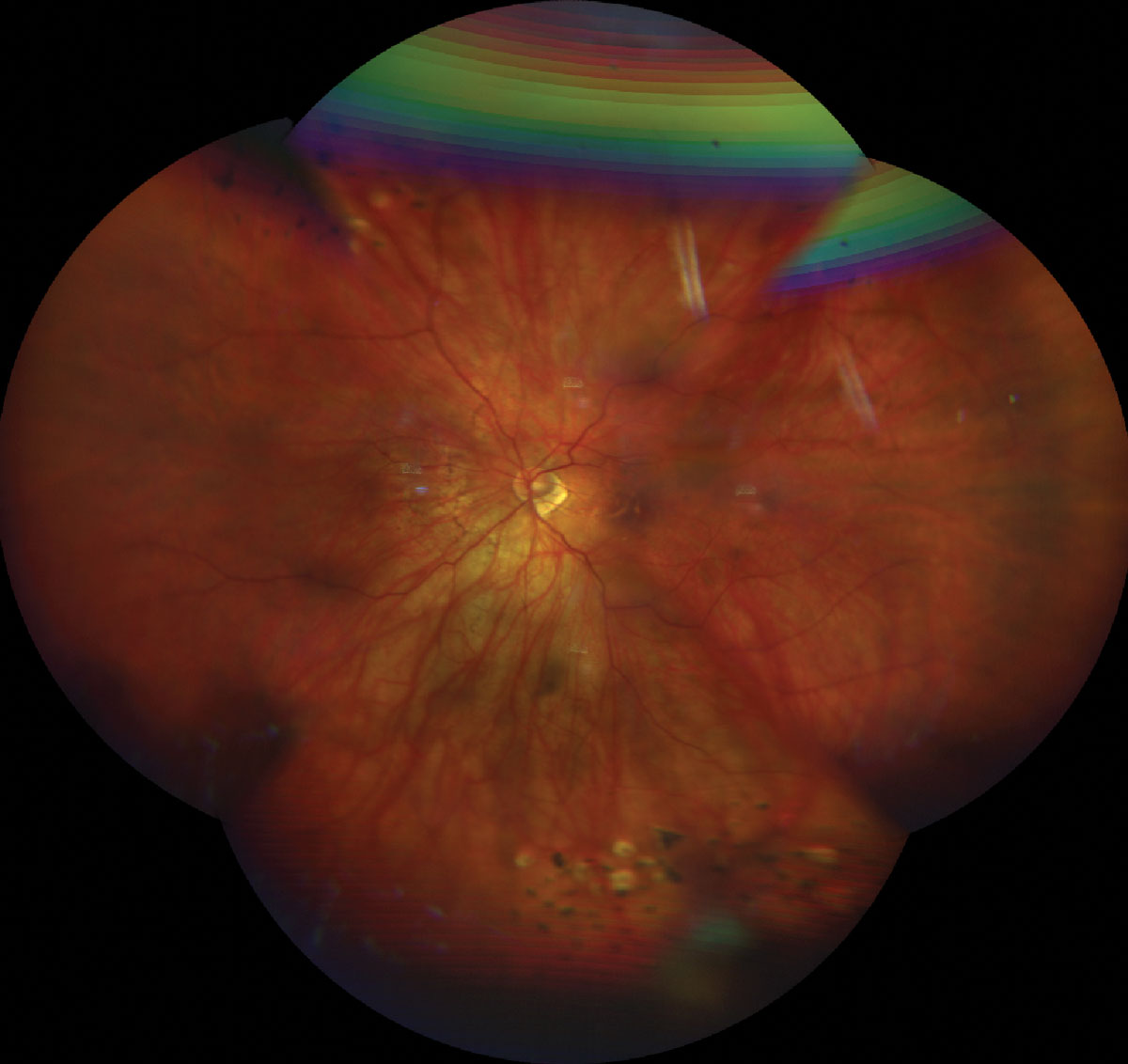
The patient had an extensive ocular history with regards to retinal changes related to PM. She underwent barrier laser retinopexy of retinal tears in both eyes approximately twelve years prior with re-treatment ten years later for additional laser barricade around the retinal tears in addition to inferior lattice degeneration in her left eye. She had documented bilateral myopic maculopathy with epiretinal membranes and right eye foveoschisis. She had no remarkable medical history; she was not taking any medications and had no known drug allergies. Family ocular history was negative.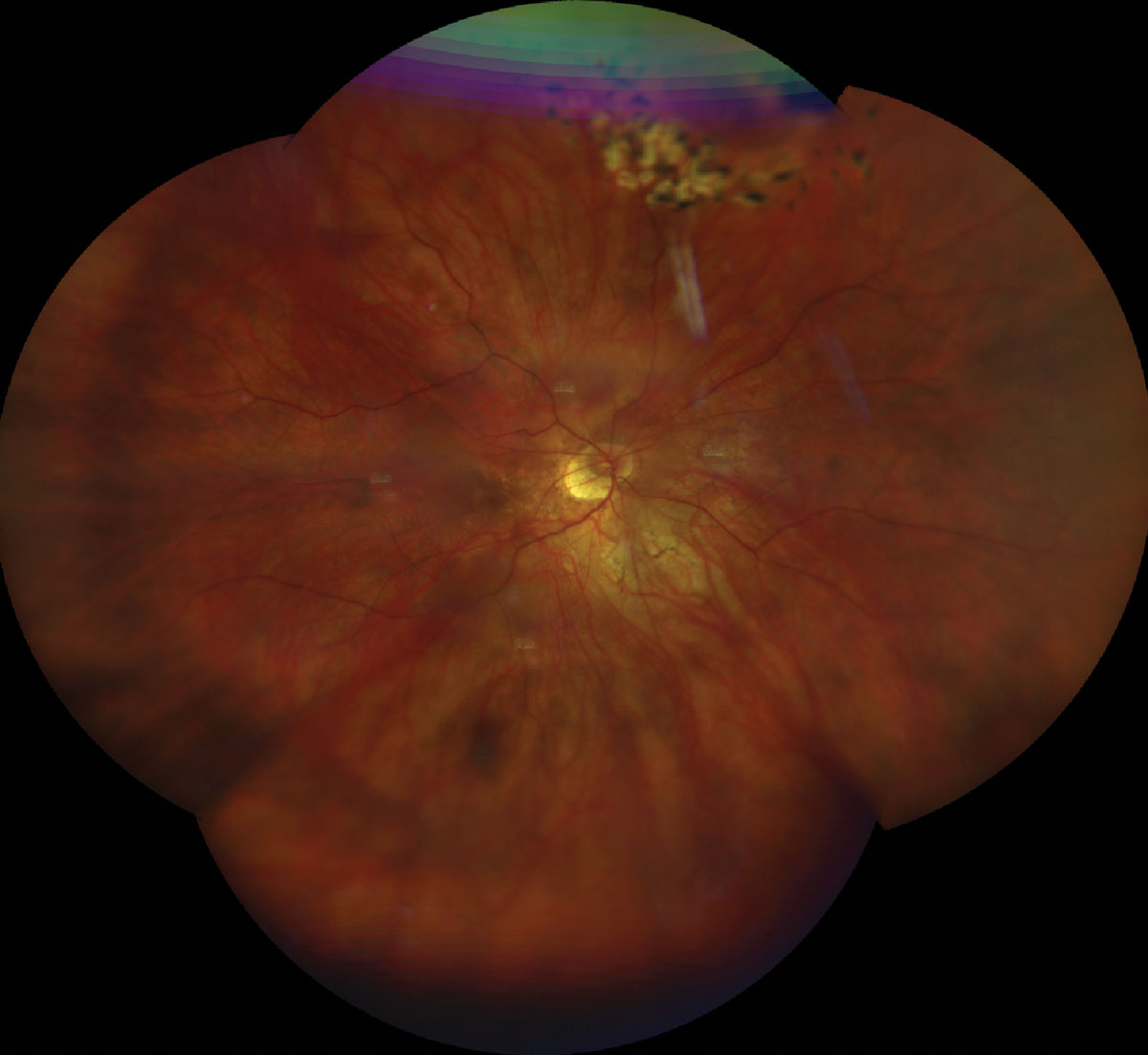
 |
|
Fig. 1. UWF retinal photo of the top (A) and bottom (B) eye showing a tessellated fundus, tilted nerve with temporal crescent. Laser scars surrounding a laser tears superior OD/OS and lattice degeneration inferior OS. Click image to enlarge. |
Best corrected distance visual acuity (BCVA) was 20/30-2 OD, 20/40+1 OS, 20/25-2 OU, PHNI at distance and 20/25 OU at near with progressive lens prescription of -14.50 2.00x008 OD and -13.00-2.00x170 OS, +2.25 add OU. Extraocular motilities, confrontation visual fields and pupils were all unremarkable. Blood pressure measured 142/83. Intraocular pressure (IOP) was 20mmHg OD and 21mmHg OS via Goldmann applanation tonometry. Evaluation of the anterior segment revealed bilateral trace nuclear sclerosis and 2+ posterior subcapsular cataracts. The previously reported posterior pole findings appreciated via dilated fundoscopy were as follows: treated retinal tear with old cryotherapy and new barrier laser scars (12:30) in the right eye, and treated retinal tears (11:00, 1:30) with pigmented lattice degeneration (5:00-6:00), all surrounded by barrier laser in the left eye. There was a small amount of subretinal fluid underlying the retinal tears that did not extend beyond the newer laser scars, otherwise there was no new breaks or detachments with scleral indentation performed 360 in each eye. Additionally, the tessellate, tigroid appearing fundus had myopic pigment changes with no foveal light reflex. Optic nerve heads (ONH) had a myopic tilt, extensive peripapillary atrophy temporally and a cup-to-disc ratio of 0.15 right eye and 0.25 left eye without evidence of clinic nerve fiber layer defects. Posterior vitreous detachment and 2+ floaters were observed in both eyes.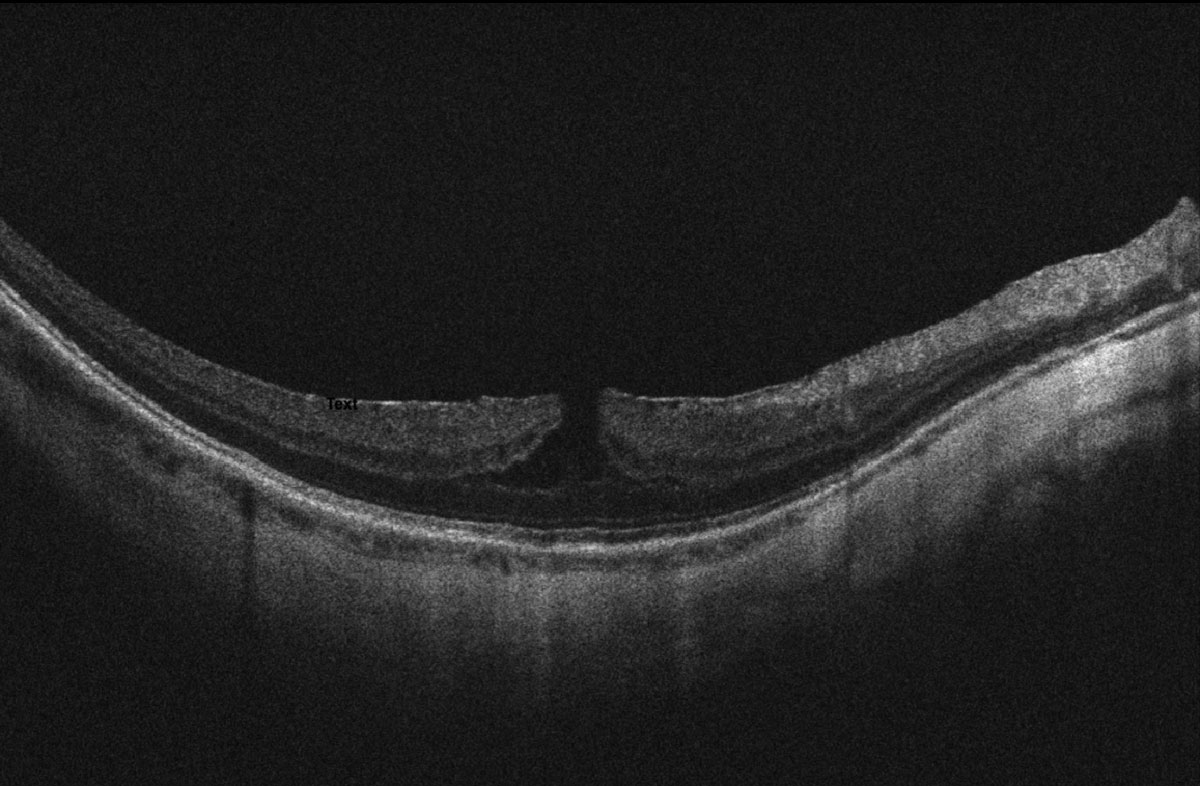
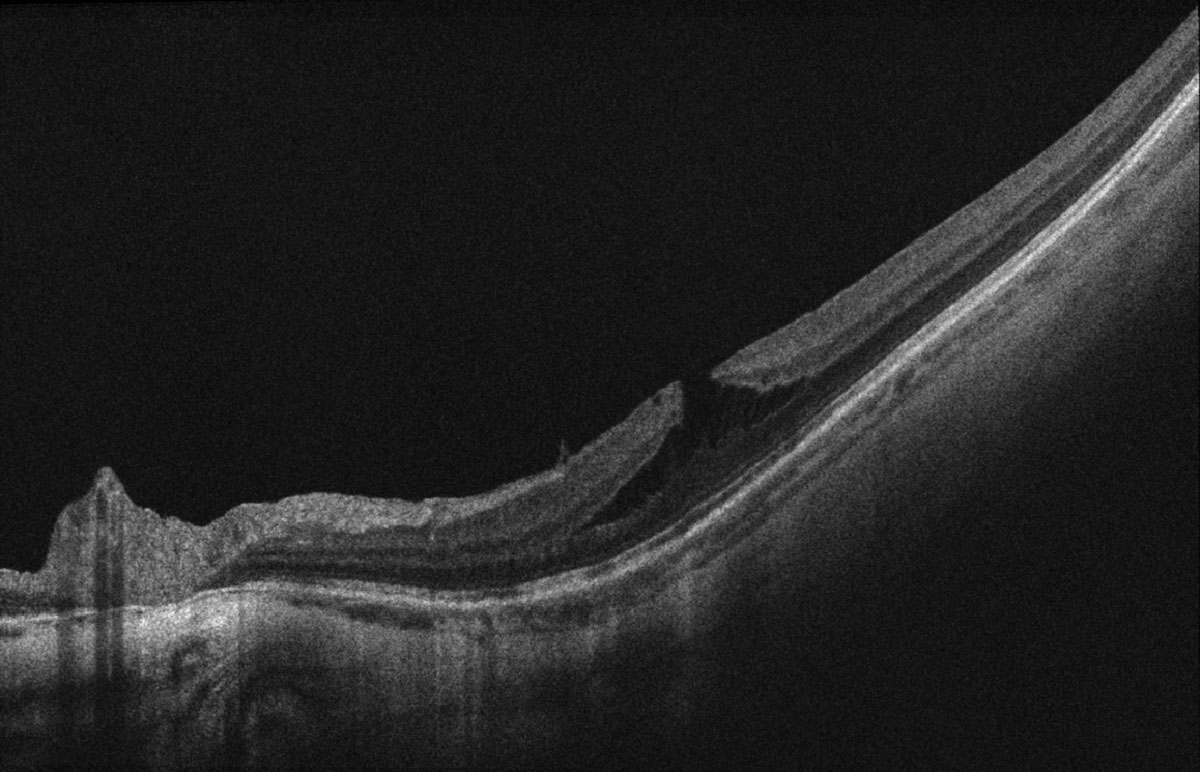 |
| Fig 2. OCT scan of the top (A) and bottom (B) macula showing ERM, myopic foveoschisis with associated lamellar macular hole OS>OD and thin choroid. Click image to enlarge. |
Ultra-widefield retinal imaging was obtained to document the retinal findings (Figures 1a, 1b). Ocular coherence tomography (OCT) scans of the macula revealed myopic tractional foveoschisis with associated lamellar hole more significant in the left eye than the right eye, and an epiretinal membrane was present bilaterally (Figures 2a-c). There was no evidence of subretinal fluid. Both eyes had a thin choroid without signs of myopic choroidal neovascularization (CNV). Upon comparison with macular cube OCT change analysis taken five months prior, the right eye appeared stable (-4μm change centrally, Figure 3a), while an increase in subfield thickness of +22μm was reported the left eye (Figure 3b).
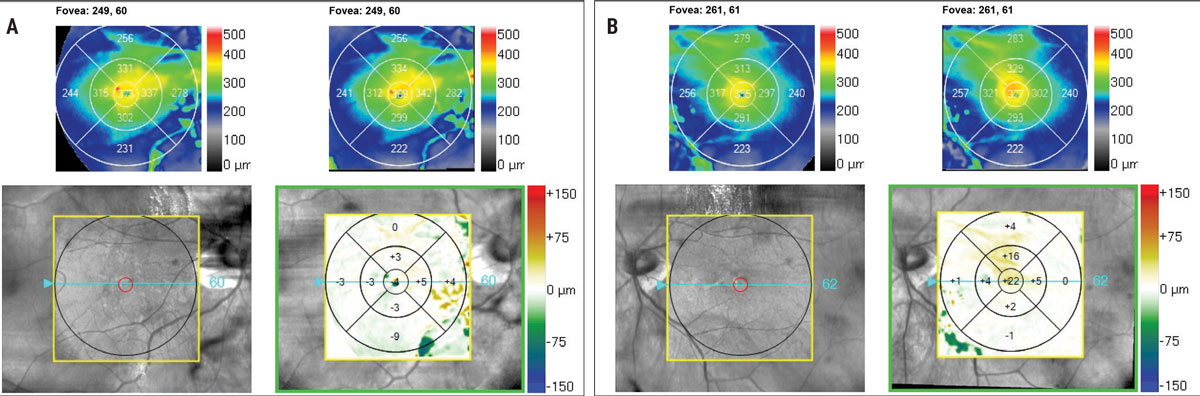 |
Fig 3. OCT change analysis of the macula taken 6 months apart. The right eye (A) is stable, the left eye (B) shows evidence of +22μm central subfield thickness. Click image to enlarge. |
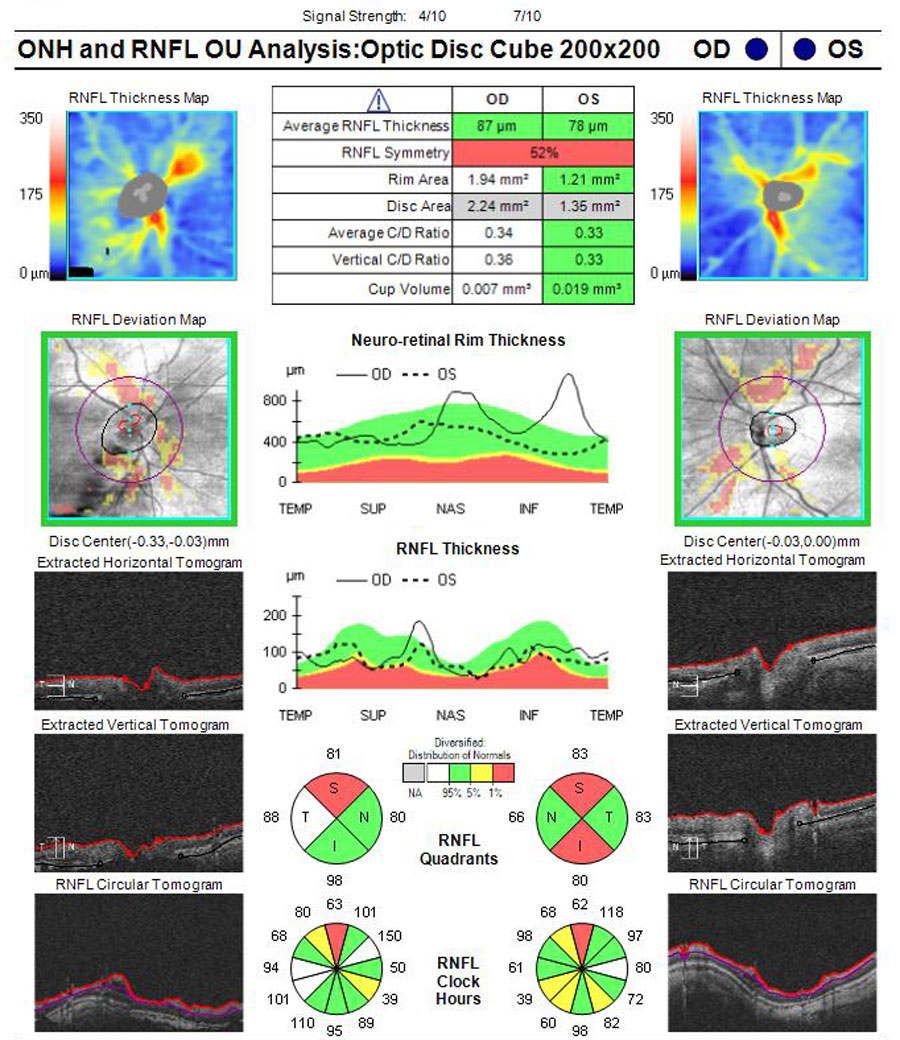
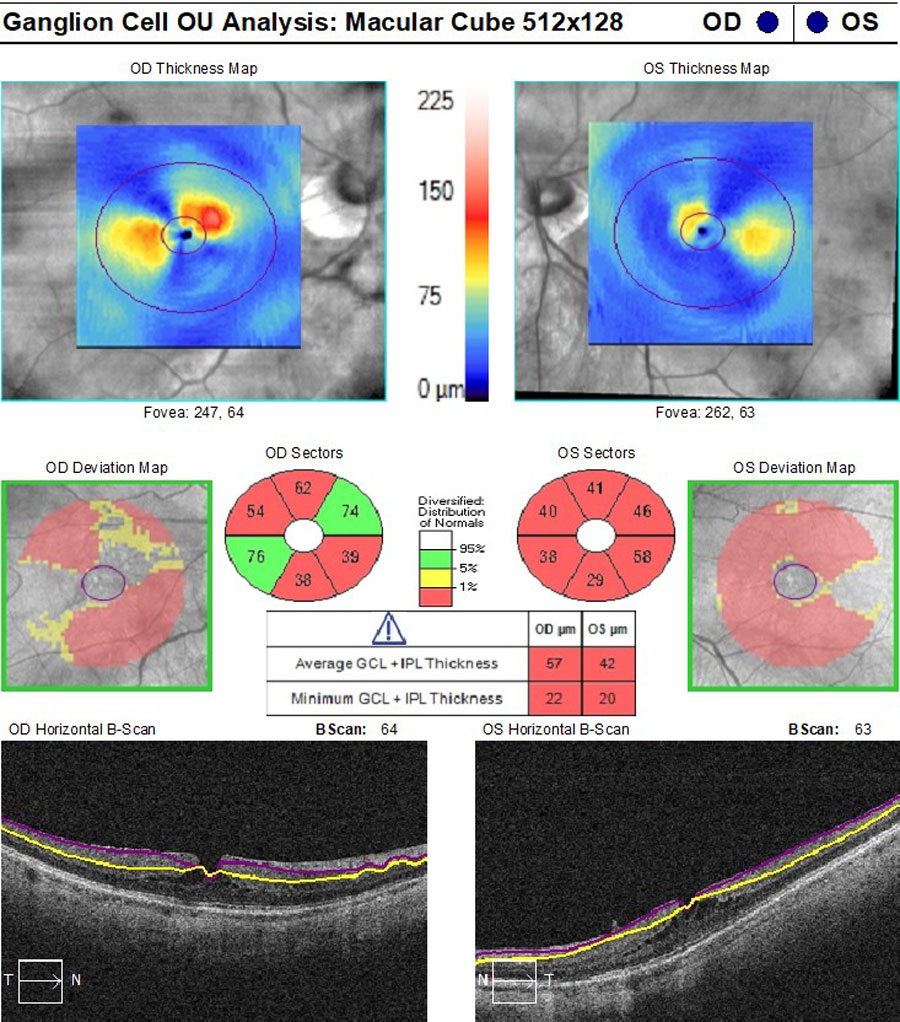
ONH retinal nerve fiber layer (RNFL) and ganglion cell complex (GCC) OCT scans were acquired due to borderline high IOPs and PM. ONH scans had segmentation errors as a result of myopic tilt but there was evidence of superior RNFL loss in both eyes (Figure 4), stable in the right eye but possible progression in the left eye when compared with the previous scans taken three years before. GCC was diffusely thin but without evidence of continued loss on three scans over one year on progression analysis, with particular focus on the temporal macula area of concern with glaucoma (Figure 5).
 |
|
Fig 4. ONH OCT scans showing poor segmentation with PM nerves. Possible thin RNFL superior OD and superior/inferior OS. Click image to enlarge. |
At the current visit, the findings including the natural course and visual prognosis related to PM degeneration were discussed with the patient. She was referred back to her retinal ophthalmologist given the change in left macular thickness to evaluate whether surgical intervention with pars plana vitrectomy or observation is recommended. Additionally, she was given an Amsler grid for at-home visual monitoring and educated regarding the symptoms of a retinal detachment, including flashes of light, floaters or a black curtain over her vision, and advised to immediately return to the clinic or urgent care if experienced. The patient was instructed to return to the retina clinic in three months for glaucoma workup testing, including pachymetry, gonioscopy, IOP, and Humphry visual field testing.
The report from retinal ophthalmology appointment recorded similar clinical findings with no surgical intervention recommended at this time with continued observation.
Discussion
Pathologic myopia entails a diverse collection of degenerative ocular manifestations,” Cappellani explained. “Parolini et al. proposed an anteroposterior push-pull “Game of Forces” played by the vitreous liquefaction and contraction verses sclera elongation and enlargement, subsequently wreaking havoc on the retina. Consequential changes induced by pathologic myopia are responsible for macular, peripheral and optic disc alterations, as well as associations with glaucoma and cataracts. These structural complications, especially those involving the macula, cause severe vision loss in patients with the disease. The diagnostic entities under the umbrella of PM are discussed below.
I. Posterior Fundus Shape Changes
I.a. Posterior staphyloma
Posterior staphyloma is a local bulging of the sclera at the posterior pole of the eye with a radius less than the surrounding curvature of the eyewall.12 The presence of a posterior staphyloma is considered a hallmark lesion of PM with an increased prevalence in older age groups. One study reported posterior staphyloma was observed highly myopic eyes in 2.7% aged 35 to 50 years, 9.2% in 50 to 59 years, 21.2% in 60 to 69 years and 43.6% in aged 70 to 79 years. OCT, particularly longer wavelength enhanced depth imaging (EDI)-OCT, offers clear visualization of the characteristic concavity of the posterior globe with adjacent thinned choroid. It is important to note that scleral ectasia can also occur in non-myopic eyes and therefore it cannot be a definitive diagnostic feature of the condition. Nonetheless, it has been well established that posterior staphylomas indicate more severe alterations and a higher risk of myopic maculopathy.14
I.b. Dome-shaped macula
A dome-shaped macula is a distinct convex elevation of the macula typically housed within a posterior staphyloma. While difficult to appreciate on fundus examination, EDI-OCT or B-scan ultrasonography facilitates the understating that the protrusion is a result of subfoveal scleral thickening. The pathogenesis remains unclear, but it has been suggested the dome aids in relieving traction on the fovea, thereby preventing myopic foveoschisis.15
 |
|
Fig 5. GCC OCT scans showing poor segmentation resulting in diffusely thin GCC measurements but no characteristic temporal macular GCC loss. Click image to enlarge. |
II. Myopic Maculopathy
II.a. Myopic macular lesions
The meta-analysis for pathologic myopia (META-PM) developed a consensus on the classification of myopic maculopathy which involved 5 categories: (0) no macular lesions, (1) tessellated fundus, (2) diffuse chorioretinal atrophy, (3) patchy chorioretinal atrophy, and (4) macular atrophy. This grading was modelled after long-term observation of and an increased risk of developing myopic CNV. Three additional characteristic “plus lesions,” lacquer cracks, myopic CNV, and Fuchs spot, do not fit into a particular category, rather can develop from or coexist at any time throughout the course of disease.12 Building onto that classification system, ATN staging—atrophy, traction and neovascularization—provides supplemental information regarding the tractional component with five stages of inner/outer foveoschisis, foveal detachment, macular hole and retinal detachment (RD).14
II.b. Myopic traction maculopathyTraction exerted by adherent posterior hyaloid on the internal limiting membrane in highly myopic eyes generates vitreomacular interface alterations, termed myopic traction maculopathy, is exacerbated in the presence of posterior staphyloma and epiretinal membrane. The wide variety of pathologies can affect up to 30% of PM eyes.11 Myopic foveoschisis is a dynamic condition involving the separation of retinal layers, which remain connected by Muller cells.14 The multifactorial condition is mainly attributed to traction and may remain stable (including stable BCVA) during long periods. However, due to the progressive nature of the condition, a lamellar or full-thickness macular hole, or foveal detachment can develop, inevitably leading to substantial visual impairment.10 Besides BCVA, OCT imaging is key to establish initial diagnosis and monitor progression. Patients who experience evidence of reduced BCVA and worsening anatomical changes on OCT are candidates for interventional management. Surgical techniques to release posterior hyaloid traction involve pars plana vitrectomy with or without gas tamponade, and often ILM peeling in cases of macular hole.14
II.c. Myopic choroidal neovascularization
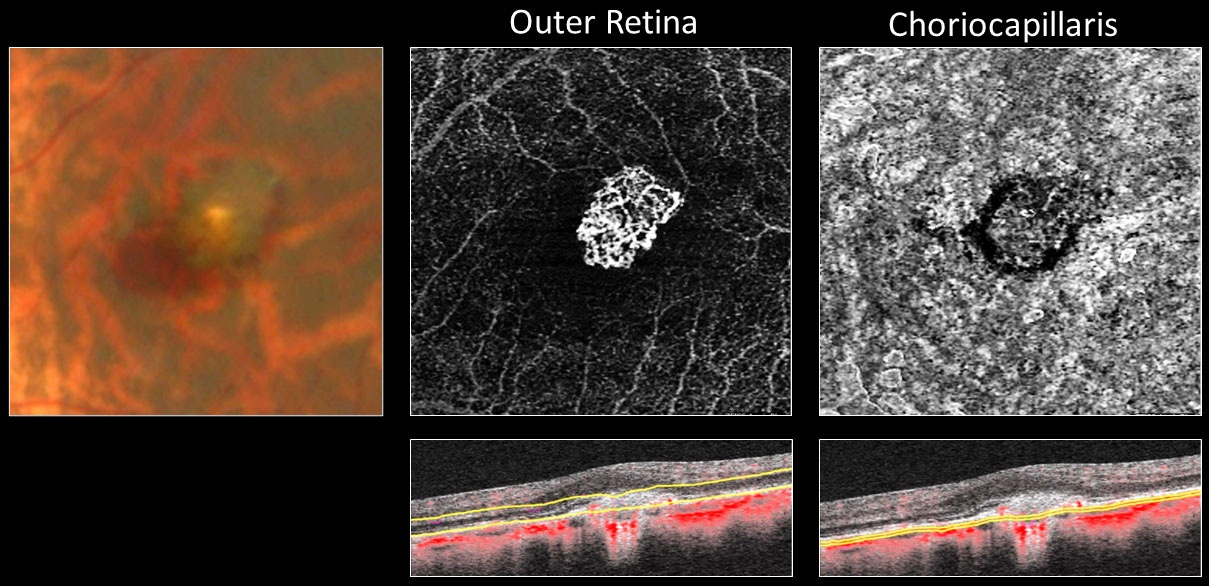
CNV is a leading cause of vision loss in PM, affecting 5% to 10% of eyes with the condition. While age-related macular degeneration is the most common etiology of CNV, myopic CNV is responsible for approximately 60% of CNV in patients younger than 50 years of age.10 This serious complication affects central vision and is commonly bilateral. Myopic CNV is associated with lacquer cracks; however, there is evidence of vascular endothelial growth factor (VEGF) is released in response to RPE cell exposure to extracellular matrix components finds a channel to the choroid via ruptures in Bruch’s membrane resulting in active choroidal CNV. Hyperpigmented Fuch’s spot represents scar formation and degenerative chorioretinal atrophy develops as the macula becomes atrophic, which drives dramatic BCVA loss. OCT and OCT-angiography (OCTA) identify characteristics of retinal thickening, subretinal elevation with fluid and CNV blood-flow observed with myopic CNV. Fundus autofluorescence is helpful for capturing hyperfluorescent lipofuscin in sick RPE. While more invasive, myopic CNV can be confirmed with fluorescein angiography showing classic hyperfluorescence in early phase and late phase leakage. Indocyanine green is sometimes useful in cases of dense macular hemorrhages due to its ability to penetrate through blood. Once the diagnosis of myopic CNV is made, prompt management with intravitreal anti-VEGF therapy is the gold standard treatment option.14 The importance of distributing an Amsler grid for at-home monitoring of visual changes between follow-up visits should never be overlooked.
 |
|
Fig 6. Fundus photo, OCT and OCT-A imaging of myopic CNV in a 44-year-old female who was -10D OU prior to LASIK. Click image to enlarge. |
IV. Peripheral retinal changes
Expanding the focus beyond the macula, peripheral retinal changes seen in PM include lattice degeneration, retinal holes, retinal tears, all of which can lead to RD. Higher levels of myopia is positively correlated with an increased risk of RD with odds ratio (OR) of 3.45 for low (-0.50 to <-3D), 8.74 for moderate (-3 to <-6D), and 12.62 for high myopia (<-6D).16 UWF imaging compliments dilated fundus examination for investigative and documentation purposes. Given the greater chance of lattice degeneration progressing to RD, prophylactic laser photocoagulation is recommended in highly myopic eyes. RD repair options include scleral buckle and PPV with or without tamponade, wherein scleral buckling is preferred in younger individuals with lower rates of secondary cataract development.10 Education regarding continuous monitoring and seeking prompt treatment in wake of RD symptoms is crucial to discuss amongst patients with PM.
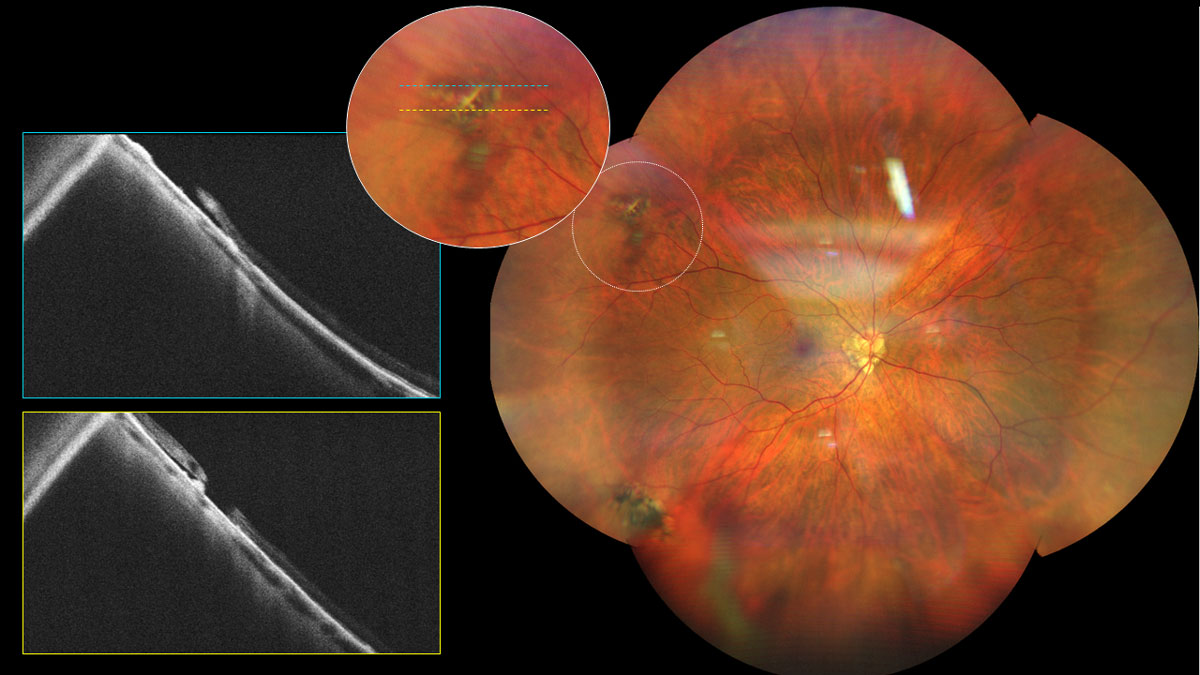 |
|
Fig 7. UWF and OCT imaging of a myopic patient with lattice degeneration and chronic RD. Click image to enlarge. |
V. Optic disc changes
Mechanical stretching of the globe in PM fabricates changes in the optic nerve and peripapillary region. Papillary and peripapillary areas are distorted, resulting in scleral crescents, tilted discs and acquired megalodiscs.10 With high degrees of myopia, peripapillary atrophy can circumferentially surround the entire disc which is thought to cause glaucomatous visual field defects. In association with the enlarged optic disc and Bruch’s membrane opening, the lamina cribrosa thins and elongates, subsequently decreasing the relative distance between intraocular and retrobulbar components. This posterior shift creates a rise in translaminar cribrosa pressure gradient between IOP and cerebrospinal fluid pressure causing subsequent axonal insult, coined myopia-associated glaucomatous optic neuropathy. Whether the PM nerve damage merely resembles structural loss in glaucoma, as opposed to coexists with glaucoma is diagnostically difficult. The diminished pink nerve colour, bright peripapillary atrophy and stretched neuroretinal rim contribute to complex cup/disc outlining and segmentation errors with OCT scans.17 Nevertheless, myopia is a well-established risk factor for glaucoma and numerous studies have demonstrated a higher prevalence of glaucoma with higher levels of myopia, therefore careful attention to IOP and intrapapillary vessel kinking close to the optic disc border are important for glaucoma detection and progression. Furthermore, outer visual field defects can help in situations where physiological and morphometric techniques fail.10,17
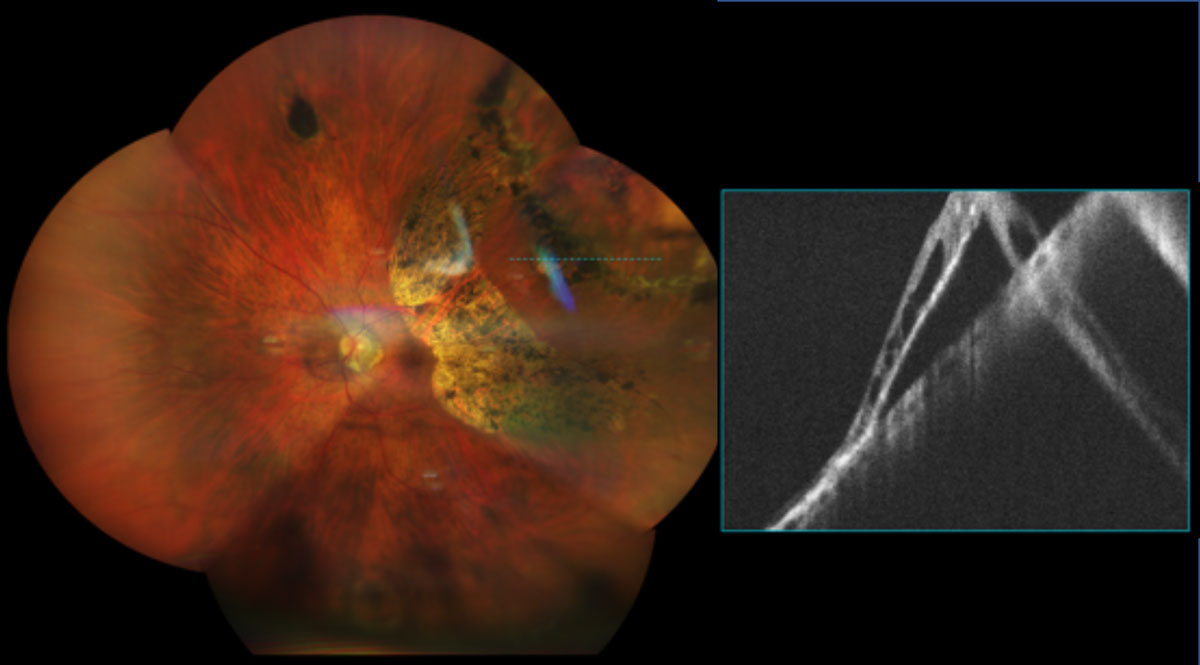 |
|
Fig 8. UWF and OCT imaging of a 65-year-old female -9.50D myope with lattice degeneration and chronic RD s/p barrier laser retinopexy. Click image to enlarge. |
Conclusion
PM is a growing cause of visual impairment and has significant socioeconomic impact worldwide. An armamentarium of multimodal imaging is vital for the diagnosis and management of posterior segment structural changes that occur secondary to excessive axial elongation. Patients must be educated on the necessity of consistent monitoring and seeking immediate care in the presence of symptoms for these associated sight-threatening complications. Anti-VEGF therapies and vitreoretinal surgery have been shown to be effective mitigating strategies; however, vision loss can be devastating. The rising prevalence rates of myopia implies the associated ocular diseases will follow suit, shedding light on evidence-based preventative measures, which could ultimately lead to a reduction of patients with pathologic myopia.
Thank you to Dr. Carolyn Majcher for her help with the case report and spreading her knowledge and passion for retinal diseases.
1. Chua J, Wong TY. Myopia—the silent epidemic that should not be ignored. JAMA Ophthalmol. 2016;134(12):1363-4. 2. Holden BA, Fricke TR, Wilson DA, et. al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42. 3. Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci. 2019;96(6):463-5. 4. Holy C, Kulkarni K, Brennan NA. Predicting costs and disability from the myopia Epidemic – a worldwide economic and social model. Invest Ophthalmol Vis Sci. 2019;60(9):5466. 5. Vagge A, Ferro Desideri L, Nucci P, et al. Prevention of progression in myopia: a systematic review. Diseases. 2018;6(4):92. 6. Mutti DO, Hayes JR, Mitchell L, et. al. Refractive error, axial length and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510-9. 7. Flitcroft DI, He M, Jonas JB, et al. IMI–Defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20-30. 8. Holden B, Sankaridurg P, Smith E, et al. Myopia, an underrated global challenge to vision: where the current data takes us on myopia control. Eye. 2014;28(2):142-6. 9. Verkicharla PK, Ohno‐Matsui K, Saw SM. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt. 2015;35(5),465-75. 10. Raval N, Kang JJ, Kim YH. A review of pathologic myopia. Eye Contact Lens. 2022;48(10):403-9. 11. Parolini B, Palmieri M, Finzi A, et al. Myopic traction maculopathy: a new perspective on classification and management. Asia Pac J Ophthalmol. 2021;10(1):49-59. 12. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Meta-Analysis for Pathologic Myopia (META-PM) Study Group. Am J Ophthalmol. 2015;159(5):877-83. 13. Numa S, Yamashiro K, Wakazono T, et al. Prevalence of posterior staphyloma and factors associated with its shape in the Japanese population. Scientific Reports. 2018;8(1):4594. 14. Ruiz-Medrano J, Montero JA, Flores-Moreno I, et al. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80-115. 15. Lee GW, Kim JH, Kang SW, Kim J. Structural profile of dome-shaped macula in degenerative myopia and its association with macular disorders. BMC Ophthalmol. 2020;20(1):1-9. 16. Haarman AE, Enthoven CA, Tideman JW, et al. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. 17. Ohno-Matsui K., et al. (2021). IMI pathologic myopia. Invest Ophthalmol Vis Sci. 2021;62(5):5. |

