A 70-year-old white male presented with a history of visual distortion in his left eye. Best-corrected visual acuity measured 20/20 O.D. and 20/50 O.S. Amsler grid testing was positive for metamorphopsia O.S.

Spectral domain optical coherence tomography (SD-OCT) imaging of the left eye revealed the presence of vitreomacular traction syndrome (VMT).
The posterior hyaloid membrane appeared as a moderate, reflective band in a “V-shaped” configuration that was partially detached nasal and temporal to the central macula with a continuous attachment to the foveal area. Based upon these findings, we referred the patient to a retinal specialist for a surgical consultation.
VMT
A posterior vitreous detachment (PVD) is common in elderly individuals. Although PVD is believed to be benign in most cases, it may contribute to tractional vitreoretinal conditions at the site of firm interfaces.
Vitreoretinal traction plays an integral role in several retinal conditions, including VMT. VMT is characterized as an incomplete detachment of the posterior vitreous hyaloid with persistent adherence to the macula. The natural course of the disease is variable, but most cases will progress and cause further structural and functional loss.
Pars Plana Vitrectomy
Today, the role of the vitreous in the pathogenesis of VMT is well known. Because vitreoretinal interface adherence plays a role in the pathogenesis of VMT, the primary treatment goal requires complete separation of the posterior hyaloid from the internal limiting membrane (ILM).
A complete spontaneous PVD has been reported in just 10% of cases.1-3 So, pars plana vitrectomy (PPV) is often necessary to alleviate the primary macular traction. The literature reports a 75% success rate of anatomical restoration following PPV.4-6 PPV has been used for more than four decades and despite the evolution of better surgical tools and techniques, a complete removal of the vitreoretinal interface is not always guaranteed.7
Pharmacological Vitreolysis
PPV can be taxing and an attempt to remove a strong vitreoretinal adherence may yield several complications, including cataract formation, rhegmatogenous retinal detachment (RRD), endophthalmitis and retinal hemorrhages, as well as elicit the formation of other maculopathies. For this reason, several recent studies evaluated the use of pharmacological agents to aid in the dissection of the vitreoretinal interface.8-10 Building on these studies’ results, researchers have begun to evaluate safer, more efficient treatment options, such as pharmacological vitreolysis, to treat patients with VMT.
A complete PVD is the result of vitreous liquefaction and concomitant vitreoretinal adhesion compromise. Pharmacological vitreolysis is an emerging treatment modality that is being explored to induce PVD.
Pharmacological vitreolysis involves the use of enzymatic agents to alter the biochemistry of the vitreous, resulting in complete cleavage of the vitreoretinal adhesion.8-10 An ideal enzymatic agent would be responsible for causing vitreous liquefaction simultaneously with the mechanical induction of vitreoretinal separation. Experimental and clinical studies have confirmed the benefits of a variety of agents––such as dispase, chondroitinase and hyaluronidase––in manipulating the vitreous to create a complete PVD.8-10
Microplasmin
One of the most promising emerging enzymatic agents is microplasmin (ThromboGenics), which has shown great potential in both animal and human trials.12-15 Microplasmin is an active, recombinant, DNA-derived component of plasmin. It functions as a thrombolytic agent that is capable of hydrolyzing vitreous glycoprotein. This small molecule can easily penetrate the epiretinal tissue and, by compromising the vitreous’ biologic “glue,” cause cleaving at the vitreoretinal interface.9 Clinical studies of microplasmin have demonstrated a complete PVD in both animal model and postmortem human eyes.9,12
The recently published Phase III MIVI-TRUST (Microplasmin for IntraVitreous Injection––Traction Release without Surgical Treatment) study demonstrated positive, statistically significant results for the potential of microplasmin to be used to treat VMT.14,15 The MIVI-TRUST study consisted of two multi-center, randomized, placebo controlled, double-masked trials. Both trials met primary endpoint, achieving the non-surgical release of the focal vitreomacular junction by the first month.
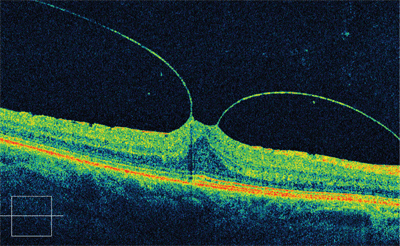
Spectral domain optical coherence tomography (SD-OCT) imaging of our patient’s left eye revealed the presence of vitreomacular traction syndrome (VMT). Would he benefit from pharmacological vitreolysis?
More than 600 subjects with VMT participated in the MIVI-TRUST study. Approximately two-thirds of the patients received an 125µg intravitreal microplasmin injection, and the remaining received a sham injection. The pool data results of both trials showed that one-fourth of the treated subjects had a complete resolution of VMT that was associated with a complete PVD, compared to just 10% of the placebo group.14,15 Visual acuity improvement was also noted among patients who were treated with an intravitreal microplasmin injection. Microplasmin was well tolerated and not associated with any retinal toxicity or retinal damage.
Pharmacological vitreolyisis has been shown to effectively manipulate the vitreous, causing a detachment of the vitreoretinal interface. This technique may offer a new treatment modality for a constellation of vitreoretinal conditions, including proliferative diabetic retinopathy, rhegmatogenous retinal detachment and macular hole, without the increased risk of surgery.
Additional benefits may include surgical adjunct, facilitating a complete PVD following PPV. In addition, this technique may decrease operating time and permit the use of smaller instruments, thereby minimizing associated complications. Finally, enzymatic agents may allow retinal specialists to manage a wide range of vitreoretinal conditions prophylactically, which could help limit progression prior to the onset of functional damage.
1. Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1995 Jan;119(1):55-61.
2. Koerner F, Garweg J. Vitrectomy for macular pucker and vitreomacular traction syndrome. Doc Ophthalmol. 1999;97(3-4):449-58.
3. Sulkes DJ, IP MS, Baumal CR, et al. Spontaneous resolution of vitreomacular traction documented by optical coherence tomography. Arch Ophthalmol. 2000 Feb;118(2):286-7.
4. McDonald HR, Johnson RN, Schatz H. Surgical results in the vitreomacular traction syndrome. Ophthalmology. 1994 Aug;101(8):1397-402; discussion 1403.
5. Smiddy WE, Green WR, Michels RG, de la Cruz Z. Ultrastructural studies of vitreomacular traction syndrome. Am J Ophthalmol. 1989 Feb 15;107(2):177-85.
6. Melberg NS, William DF, Balles MW, et al. Vitrectomy for vitreomacular traction syndrome with macular detachment. Retina. 1995;15(3):192-7.
7. Machemer R. Vitrectomy: a pars plana approach. Technical improvements and further results. Trans Am Acad Ophthalmol Otolaryngol. 1972 Mar-Apr;76(2):462-6.
8. Trese MT. Enzymatic vitreous surgery. Semin Ophthalmol. 2000 Jun;15(2):116-21.
9. Sebag J. Pahmacologic vitreolysis. Retina. 1998;18(1):1-3.
10. Tezel TH, Del Priore LV, Kaplan HJ. Posterior vitreous detachment with dispase. Retina. 1998;18(1):7-15.
11. Wang F, Zhang X, Xu X, et al. PVD following plasmin but not hyaluronidase: implications for combination of hyluronisade, chondroitinase and plasmin. Retina. 2005 Jan;25(1):38-43.
12. Stalmans P. MIVI 2T Trial: A randomized, double-masked, clinical trial of microplasmin intravitreal injection for nonsurgical treatment of VMT. ASRS 25th annual meeting. Dec 2007. Palm Spring, Calif.
13. Gandorfer A, Rohledder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004 Feb;45(2):641-7.
14. Jumper J, Pakola S. The MIVI-007 trial. Phase III evaluation of single intravitreous injection of microplasmin or placebo for treatment of focal vitreomacular adhesion. Paper presented at the American Society of Retina Specialists Meeting; August 31, 2010; Vancouver, BC.
15. Packo K, Pakola S. The MIVI-006 trial. Phase III evaluation of single intravitreous injection of microplasmin or placebo for treatment of focal vitreomacular adhesion. Paper presented at the American Society of Retina Specialists Meeting; August 31, 2010; Vancouver, BC.

