A 50-year-old black male was receiving care for an acute onset of diffuse redness in his right eye. He had been seen by a local community doctor who diagnosed him with episcleritis and began treatment with loteprednol etabonate 0.5% (Lotemax, Bausch + Lomb) q2h.

At the initial examination (reported by the referring optometrist), his intraocular pressure measured 13mm Hg O.U. He used the medication and returned for follow-up three days later, as directed. At that time, his external eye had not improved and his IOP now measured 21mm Hg O.D. and 12mm Hg O.S. Despite the short interval of loteprednol use, the doctor believed that the patient was experiencing a steroid-related elevation in IOP and immediately switched him to a non-steroidal anti-inflammatory agent, nepafenac (Nevanac, Alcon).
Additionally, the doctor scheduled a referral to our facility three days later.
Three days later, the patient presented and reported no improvement in his redness, mild ocular discomfort or photophobia with NSAID use. Biomicroscopic inspection revealed diffuse, grade 4 external conjunctival injection. The cornea was clear, save a rare endothelial keratic precipitate. There was a small area of iris stromal atrophy. Additionally, there was a very mild anterior chamber cell and flare reaction in the involved eye. There was also relative corneal hypoesthesia. At this visit, his IOP was 26mm Hg O.D. and 13mm Hg O.S.
Because the patient had used a steroid for just three days and had stopped taking it three days prior in favor of an NSAID, it was clear that the IOP response was not due to the steroid, but rather to the inflammation. The patient’s initial diagnosis of episcleritis was immediately changed to a form of anterior uveitis. In this instance, the patient was experiencing an elevation in IOP that was disproportionate to the rather minimal uveitic response and he was exhibiting iris atrophy and corneal hypoesthesia. Considering all the findings, we diagnosed the patient with herpetic keratouveitis.
The patient was told to discontinue Nevanac and was started on difluprednate ophthalmic emulsion 0.05% (Durezol, Alcon) q.i.d. therapeutically and ganciclovir ophthalmic gel (Zirgan, Bausch + Lomb) t.i.d. prophylactically.
Upon his return three days later, the redness had dissipated completely, the anterior chamber reaction was gone and his IOP had equilibrated at 14mm Hg O.U. Because his IOP decreased with the addition of a potent steroid, it became evident that the inflammatory response (and not the previous loteprednol use) caused the elevation. Subsequently, he was tapered off all medications and continues to do well.
Durezol and Uveitis
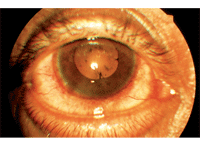
This patient presented with circumlimbal flush and broken posterior synechiae
secondary to acute anterior uveitis.
Durezol, the first topical steroid developed since 1973 for the treatment of ocular inflammation, is a difluorinated derivative of prednisolone with potent anti-inflammatory activity. The addition of two fluorine atoms to the original prednisolone molecule, as well as some other modifications, serves to increase not only the penetrance across the cornea, but also glucocorticoid receptor affinity, which enhances its anti-inflammatory ability.1 Difluprednate contains sorbic acid as its preservative.
Currently, the labeled indication for Durezol is for the treatment of postoperative inflammation and pain associated with ocular surgery. In fact, an indication for postoperative pain management makes this steroid unique. The recommended dosing regimen is one drop, two to four times daily, beginning 24 hours after surgery and continuing throughout the first two weeks of the postoperative period, followed by two times daily for a week with tapering thereafter, based upon clinical response.2 Durezol has been shown to be clinically comparable to the very potent agent betamethasone for postoperative inflammation, with an equal likelihood of a steroid response.3,4 Due to its enhanced bioavailability and clinical potency, Durezol tends to need less frequent dosing than other topical steroids.
An important feature is the emulsion vehicle, which contains the active steroid. The lipid emulsion has a smaller particle size, which increases bioavailability, provides uniform medication concentration in each drop and eliminates the need for shaking before use. In a study of dose-drop uniformity of concentration that compared Durezol to both the branded and generic versions of prednisolone acetate 1%, virtually every drop of Durezol was within 10% of the label claim, regardless of whether the bottle was stored inverted or upright, or shaken before testing.5 The concentrations of prednisolone acetate varied by bottle storage and shaking. This is especially pertinent in the management of anterior uveitis. Because the majority of prescriptions written for prednisolone acetate are filled with the generic version, great disparity in drug concentration delivery occurs if the patient doesn’t vigorously shake the bottle prior to dosing.
Currently, there are no label indications for anything other than postoperative inflammation and pain, but clinicians are utilizing Durezol for other steroid responsive conditions, such as uveitis and scleritis, because there is documented evidence of the drug’s effectiveness, particularly in recalcitrant diseases.6
In one large anterior uveitis trial of 136 patients, difluprednate q.i.d. was found to be similar to betamethasone q.i.d., and was significantly better at clearing inflammation in patients with more severe inflammation.7 Another study indicated that difluprednate administered q.i.d. was at least as effective as prednisolone acetate administered eight times/day in resolving the inflammation and pain associated with endogenous anterior uveitis.8 Most notable in this study was that no patient who was treated with difluprednate q.i.d. had to be withdrawn due to lack of efficacy and given rescue therapy (consisting of prednisolone acetate 16 times/day), whereas 12.5% of the patients being treated with prednisolone eight times/day had to be withdrawn and were given the double-dosing rescue therapy.8
Durezol provides effective treatment for anterior uveitis (as well as other steroid responsive conditions) and requires less frequent dosing than prednisolone acetate or other steroids. The incidence of steroid-related elevation in intraocular pressure is approximately 3%, which is similar to that of other topical steroids.2 Due to enhanced anti-inflammatory activity, Durezol likely can be dosed less frequently than other steroids. With conditions such as anterior uveitis, it is recommended to start with q.i.d. dosing and increase only if the clinical picture dictates, rather than starting at the higher dose frequency that typically is employed with prednisolone acetate and other steroids.
While there are yet no label indications other than for postoperative inflammation, Durezol appears to be effective in managing numerous steroid-responsive conditions, including anterior uveitis, scleritis, interstitial keratitis, herpetic keratouveitis and disciform keratitis. We have used this medication to treat the aforementioned conditions and have found great success, even in cases where more traditional steroids have failed to affect a cure.
Drs. Sowka and Kabat are consultants for Alcon. Neither author has any direct financial interest in any of the products mentioned.
1. Bikowski J, Pillai R, Shroot B. The position not the presence of the halogen in corticosteroids influences potency and side effects. J Drugs Dermatol. 2006 Feb;5(2):125-30.
2. Korenfeld MS, Silverstein SM, Cooke DL, et al. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009 Jan;35(1):26-34.
3. Jamal KN, Callanan DG. The role of difluprednate ophthalmic emulsion in clinical practice. Clin Ophthalmol. 2009;3:381-90.
4. Ohji M. Efficacy and safety results of a phase III study of difluprednate ophthalmic emulsion (DFBA), 0.05%, in postoperative inflammation. Presented at ARVO Annual Meeting, May 6-10, 2007; Ft Lauderdale, Fla.: poster B807, program 3903.
5. Stringer W, Bryant R. Dose uniformity of topical corticosteroid preparations: difluprednate ophthalmic emulsion 0.05% versus branded and generic prednisolone acetate ophthalmic suspension 1%. Clin Ophthalmol. 2010 Oct 5;4:1119-24.
6. Meehan K, Vollmer L, Sowka J. Intraocular pressure elevation from topical difluprednate use. Optometry. 2010 Dec;81(12):658-62.
7. Ohno S. Phase III non-inferiority study of difluprednate ophthalmic emulsion (DFBA), 0.05% in the treatment of anterior uveitis. Presented at ARVO Annual Meeting, May 6-10, 2007; Ft Lauderdale, Fla.: poster B808, program 3904.
8. Foster CS, Davanzo R, Flynn TE, et al. Durezol (Difluprednate Ophthalmic Emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010 Oct;26(5):475-83.

