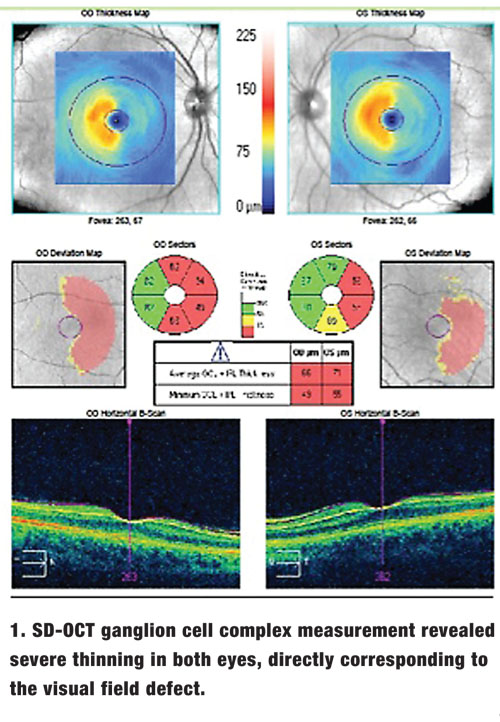
A 62-year-old white male presented to our office for visual field testing after experiencing diminished right-field vision following a stroke.
Apart from the cerebrovascular accident, which affected the left side of his brain, he had no other neurological complaints and no history of trauma, headaches or head injury. Past medical history was additionally remarkable for hypertension, being controlled by a beta blocker. His past ocular history was unremarkable. Best-corrected visual acuity was 20/25 OU.
Ocular motilities were smooth and showed full range of motion. There was no afferent pupillary defect in either eye. Slit-lamp examination revealed only mild nuclear sclerosis OU. Applanation tonometry was 15mm Hg OU. Dilated fundus exam revealed normal physiological cupping with pink, distinct neuroretinal rims. Humphrey Visual Field Analyzer 24-2 testing (Zeiss) revealed a right homonymous hemianopsia. SD-OCT ganglion cell complex (GCC) measurement revealed severe thinning in both eyes, directly corresponding to the visual field defects (figure 1).
OCT Analysis in Stroke Patients
An insult to the visual pathway often manifests as functional impairment and/or structural damage due to retrograde degeneration causing retinal nerve fiber layer (RNFL) atrophy.
A chiasmal tumor, for example, is frequently associated with bitemporal visual field defect and could show sectoral atrophy of the optic nerve with associated thinning of the RNFL.1
Furthermore, it is not uncommon to observe visual field defects in patients whose occipital lobes have been affected by stroke. A patient with a history of stroke often presents with a correlated hemianopic or quadranopic visual field defect. Homonymous hemianopias are synonymous with vascular-related strokes affecting the posterior cerebral artery.2
RNFL thinning has also been associated with such hemianopic visual field defects.3 Clinical cases of RNFL associated with congenital occipital lobe damage have been reported.4
Other studies have shown that RNFL thickness is significantly reduced among patients with related hemianopic visual field defects.5
The retinal ganglion cells (RGCs) encompass the retinal nerve fiber layers. The majority of these RGCs synapse at the lateral geniculate nucleus (LGN). Hence, damage in the visual pathway—including effects on the cortex—can also cause retrograde atrophy of the RGCs.
There is a general consensus that, although RGC damage can occur in patients with a history of stroke, it is usually noted years after the incident. In an animal model, RGC degeneration was documented four years following occipital lobe ablation.6
A recent small case series showed a correlation between RGC defects and visual field defects documented shortly after the occurrence of a posterior cerebral artery infarction.7
Researchers evaluated three subjects—ages 66, 68 and 71—who suffered a stroke within the previous 12 to 36 months.
GCC measurements were taken at the macula from the internal limiting membrane to the inner plexiform layer. RNFL thickness was measured and visual field perimetry was performed.
All patients had MRI that confirmed a posterior cerebral artery infarction with no other damage noted anywhere in the visual pathway.
Quintessential visual field defects, manifesting as homonymous hemianopsia, were documented in all subjects.
Interestingly, GCC analysis revealed a bilateral reduction corresponding to the subjects’ visual field defects.
Optic atrophy or RNFL defects were not clearly noted.
Given that 50% of the RGCs are found in the macula, GCC thinning directly correlates with RGC damage. Trans-synaptic degeneration at the level of the LGN may explain the short onset of noticeable localized GCC thinning associated with RGC damage.7
GCC is an objective, reliable measurement of RGC atrophy. Because changes in the GCC correlate favorably with visual field defects in patients with a history of a stroke and may be noted prior to RNFL defects, we may consider performing GCC measurements in all patients with a history of stroke. These measurements may help to further objectively monitor the patients over time.
Thanks to Brad Sutton, OD, with Indiana University School of Optometry, for contributing to this article.
1. Kanamori A, Nakamura M, Matsui N, et al. OCT defects characteristic RNFL thickness corresponding to band atrophy of the optic disc. Ophthalmology. 2004 Dec;111(12):2278-83.
2. Fujino T, Kigazawa K, Yamada R. Homonymous hemianopia: a rtrospective study of 140 cases. Neuro-ophthalmology 1986; 6: 17-21.
3. Convey A. Fact, artifact, and myth about blindsight. Q J Exp Psychol A. 2004 May;57(4):577-609.
4. Metha JS, Plant GT. OCT findings in congenital/longstanding homonymous hemianopia. Am J Ophthalmol. 2005 Oct;140(4):727-9.
5. Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic RGC loss identified by OCT. Brain. 2009 Mar;132(Pt 3):628-34.
6. Cowey A. Atrophy of RGC after removal of striate cortex in a rhesus monkey. Perception. 1974;3(3):257-60.
7. Yamashita T, Miki A, Iguchi Y, et al. Reduced retinal ganglion cell complex thickness in patients with posterior cerebral artery infarction detected using spectral-domain optical coherence tomography. Jpn J Ophthalmol. 2012 Sep;56(5):502-10.

