 |
An 82-year-old Hispanic female presented for an evaluation of new visual symptoms. She reported that the day prior, she had seen colorful horizontal lines in the left eye for several hours. The visual phenomenon eventually subsided, and her vision returned to baseline. She came to the emergency department with her son-in-law, who helped provide additional history. He attested that during his mother-in-law’s visual episode, he had noticed her left eye was temporarily “stuck” and not moving in conjunction with her right eye. He had asked her to try and track his finger, and although the right eye’s motilities were full, the left eye was unable to follow the target properly. He could not recall if the eyelid was ptotic at the time. Interestingly enough, the patient denied appreciating any diplopia. The episode of left eye immobility reportedly lasted about an hour.
Upon further questioning, the patient denied any recent headaches, weight or appetite loss, fevers or chills. She was unable to give a clear answer when asked about jaw pain with chewing (jaw claudication).
The patient had a past ophthalmic history of cataracts and glaucoma, managed with nightly latanoprost in both eyes. Her past medical history was significant for coronary artery disease, diabetes mellitus, hypertension and renal failure.
Her entering visual acuities were 20/350 OD and 20/50 OS. She had a dense cataract in the right eye, greater than the left eye, which we felt explained the reduction in visual acuity. Her intraocular pressures were 14mm Hg OD and 12mm Hg OS. Her pupils were round and reactive to light without an afferent pupillary defect, and confrontation visual fields were full to finger counting. Extraocular movements and color vision were full bilaterally. Slit lamp examination revealed mild blepharitis and the aforementioned cataracts. She had moderate optic nerve cupping consistent with glaucoma, but there was no appreciable optic nerve pallor or edema. The remainder of the posterior segment was unremarkable.
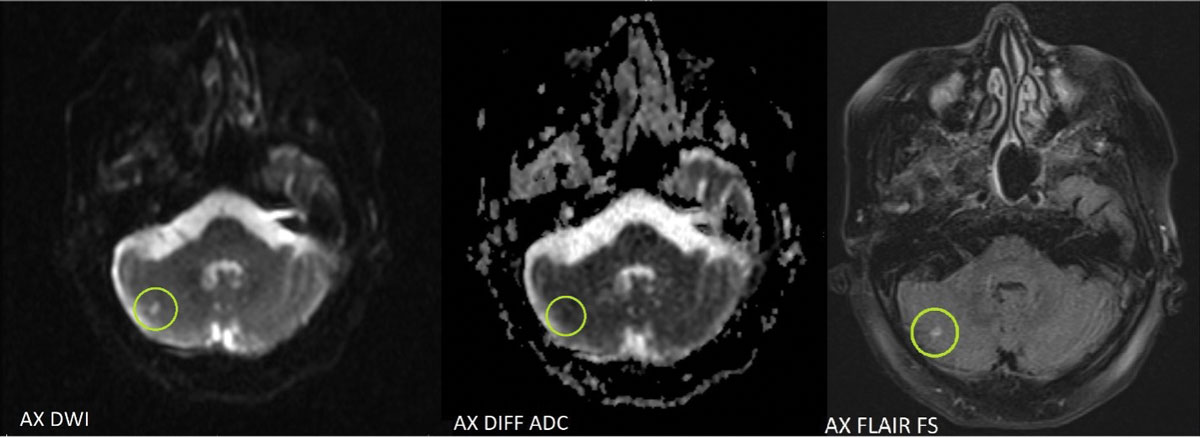 |
| Axial MRI images show acute lacunar infarct (circled in green) of the right cerebellar hemisphere. Areas that are bright on diffusion weighted images and dark on apparent diffusion coefficient are consistent with acute infarct.8 A hyperintense area can be seen on fluid attenuated inversion recovery imaging due to the presence of vasogenic edema within hours after an acute stroke.9 Click image to enlarge. |
Take it With a Grain of Salt?
On examination, her vision was 20/150 (pinhole 20/40) OD and 20/20 OS. Her intraocular pressures were 16mm Hg OD and 17mm Hg OS. The pupillary exam revealed pupils that were equal in size and no afferent pupillary defect. Her extraocular motilies were normal. Slit lamp examination revealed mild nasal injection in the right eye with a 4mm linear full-thickness corneal laceration nasally. The anterior chamber of the right eye had 1+ cell and 1-2+ pigment. There was a focal anterior cataract nasocentrally. The posterior segment exam was unremarkable, and no evidence of vitritis, intraocular foreign body or retinal damage was seen. The left eye exam was unremarkable.
As optometrists, we have a unique ability to visualize most pathology in its natural state. From macular degeneration or retinal detachments to cataracts and corneal ulcers, most of what we need to see lies right in front of our eyes. It is one of the features that likely draws many of us into this field in the first place. But in a case such as this one, in which the patient’s symptoms have resolved by the time they present to us, the clinician is faced with a difficult task. We are forced to rely solely on the patient’s narrative, which can prove challenging when we are not accustomed to doing so.
In this case, the patient provided as great of detail as she was able, but her story was tough to follow. For example, she initially said the colored lines she saw were in the left eye. Later, she reported that she had seen them in both eyes. As we all know, the laterality of such visual distortions can make a significant impact on our list of differential diagnoses. Additionally, though the patient had denied double vision, her son-in-law had been with her at the time and was able to provide a recount of her abnormal ophthalmic motilities.
So, now for our decision: do we tell the patient that everything looks good on our end, or do we pursue a further explanation for the transient, and seemingly disparate, symptoms?
During the examination, a very intentional discussion regarding her reported symptoms occurred. Despite our relatively proficient clinical Spanish, a translator was used to clearly communicate all pertinent details of the story. Ultimately, we felt her symptoms were repeatable enough to likely be reliable and true.
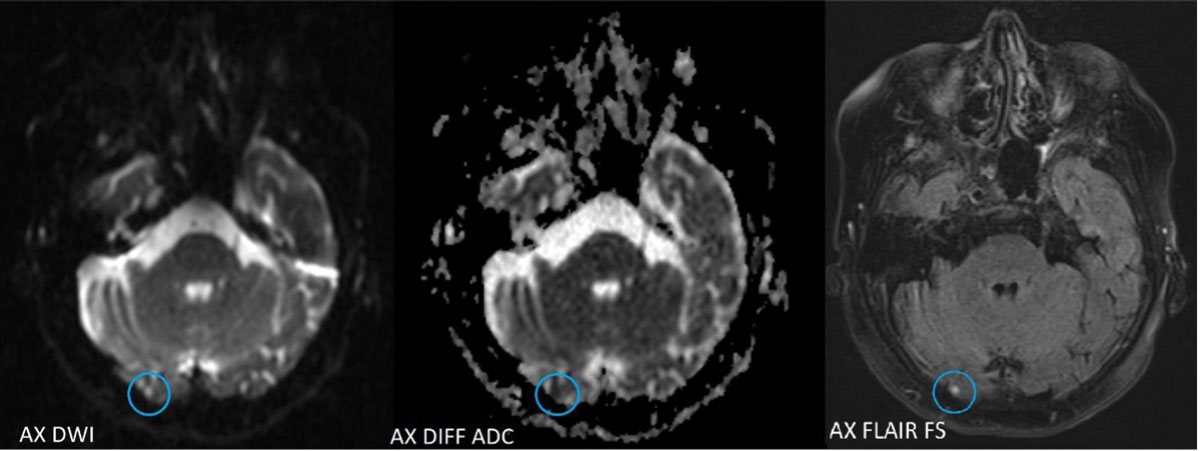 |
| Axial MRI images show acute lacunar infarct (circled in blue) of the inferior medial aspect of the lingual gyrus of the right occipital lobe. Click image to enlarge. |
Okay, You Convinced Me
Given our concern, emergent laboratory studies were ordered. The erythrocyte sedimentation rate, C-reactive protein and complete blood count with differential (including platelets) returned grossly unremarkable, essentially eliminating giant cell arteritis. MRI of the brain and orbit without contrast was also obtained. Gadolinium contrast was not administered due to the patient’s known history of renal failure and the risk of acute kidney injury or toxicity. Neuroimaging revealed a solid-appearing sellar/suprasellar mass, likely a pituitary macroadenoma, abutting the prechiasmatic segments of both optic nerves. Additionally, acute lacunar infarcts were visualized in both the lingual gyrus of the right occipital lobe and the inferior right cerebellar hemisphere.
These findings were consistent with an acute stroke, and the patient was transferred to our affiliated hospital for a full stroke workup and neurology consultation. She was diagnosed with a stroke due to embolism of the right cerebellar artery. Although a definitive source of emboli was not detected, she was started on low-dose daily aspirin, and a heart monitor patch was placed for continued observation.
Our patient was given oral levofloxacin in the emergency room on the day of her presentation, and fortified topical antibiotics, vancomycin and tobramycin were prescribed. She was also advised to start topical corticosteroids and mydriatic drops to minimize inflammation. Intravitreal antibiotics were not initiated at this point given the general lack of inflammation inside the eye. The patient was monitored closely over the following weeks and fortunately did not appear to have any increase in inflammation, pain or intraocular pressure. Cataract surgery was scheduled for one month later.
Since globe trauma increases the risk of any intraocular procedure, including cataract surgery, there are some important points to consider when planning for surgery and educating the patient on potential complications and outcomes. “Capsular integrity is of utmost importance when evaluating a patient with corneal perforation. We can assume capsular integrity is violated when cortical or lenticular material is found in the anterior chamber. Another clue is assessing for phacodonesis, as this may indicate direct trauma to the lens. An important point, however, is that it may be difficult to visualize these signs given the robust inflammation and corneal haze one may see on presentation,” says Zubair Ansari, MD, the surgeon in this patient’s case. “As a general rule of thumb, expect the unexpected when it comes to traumatic cataract. Traumatic cataracts tend to have higher rates of phacodonesis, vitreous prolapse and posterior capsular rupture.”
As far as educating the patient on postsurgical outcomes, Dr. Ansari notes that a thorough preoperative conversation about expectations is important given the higher risk of complications. These patients may often require a secondary or sulcus-placed intraocular lens, which can make refractive outcomes less predictable. Additionally, this particular patient had paracentral irregular corneal astigmatism that would likely lead to decreased visual quality even after the cataract was removed. Since the astigmatism was non-central and irregular, a toric or multifocal lens was not appropriate. The patient underwent a carefully performed cataract surgery, in which the anterior capsular violation was incorporated into the capsuolorhexis, and a standard monofocal lens was placed in the capsule. She was advised to consider a rigid gas permeable contact lens after surgery for the best visual outcome.
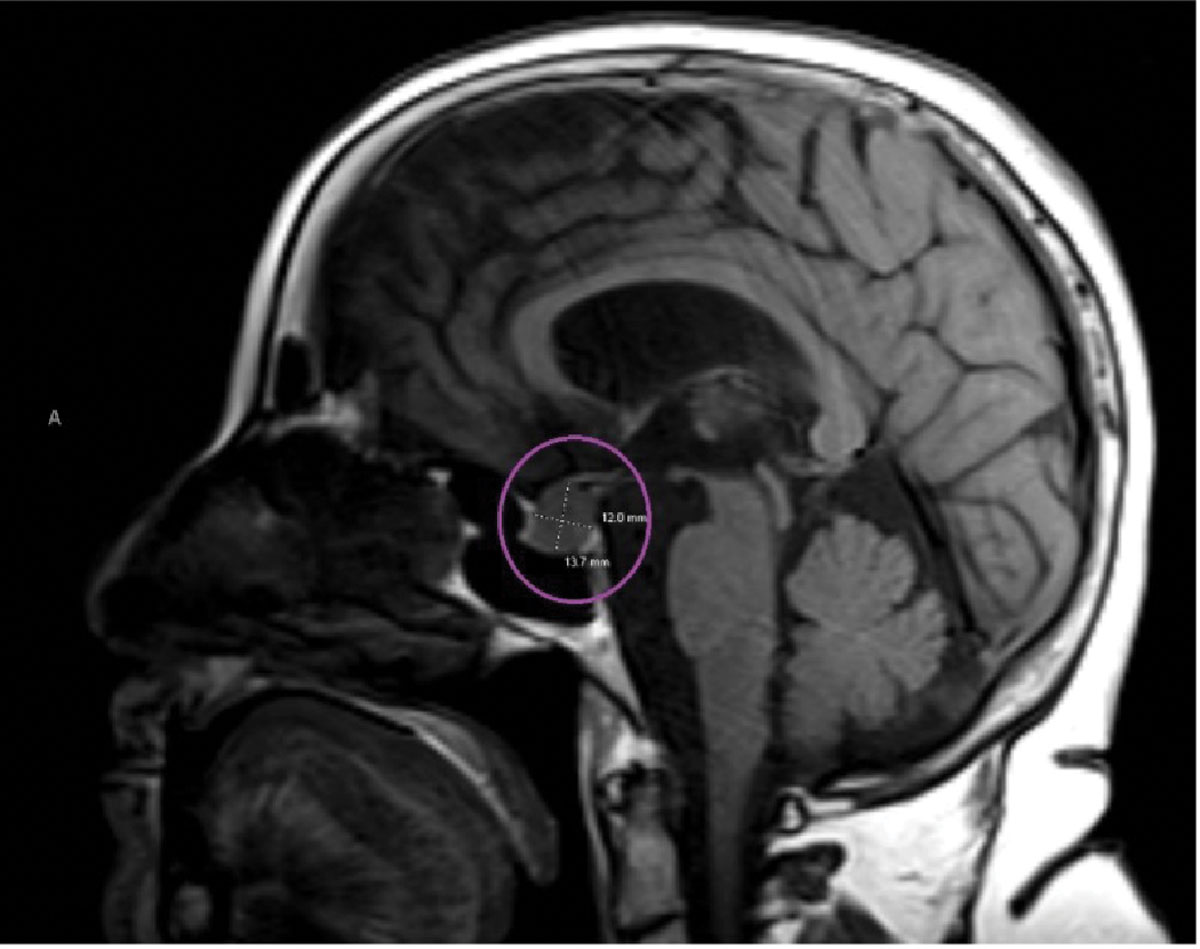 |
Sagittal MRI reveals a pituitary lesion, most likely a macroadenoma (circled in purple). It was described to be abutting both optic nerves anterior to the chiasm. Click image to enlarge. |
Doubling Down
Our patient was found to have multiple pathologies on neuroimaging that could affect the visual system. First, she had a pituitary macroadenoma. Though many pituitary adenomas are asymptomatic and found incidentally, pituitary macroadenomas (adenomas measuring >10mm) may cause loss of vision due to their size and proximity to the optic chiasm/nerves.1 They can cause monocular or binocular vision changes, but the classic presentation is a bitemporal visual field defect due to chiasmal compression.2 Headaches in the periorbital region are common, and patients may experience additional symptoms such as amenorrhea, loss of libido, galactorrhea and infertility due to excessive hormonal release in functional adenomas.3 Patients may also develop diplopia due to compression of one (or more) cranial nerves controlling ocular motilities, but motility deficits from compression would most likely persist rather than be transient as in our patient.4,5
Our patient also had evidence of acute stroke affecting the cerebellar and occipital regions. Stroke symptoms are inherently based on their extent and location. Cerebellar strokes often present with nausea, dizziness, gait ataxia, loss of balance and vomiting.6 She denied any of these symptoms, but the cerebellum is also involved in oculomotor control, and therefore, patients with cerebellar lesions may present with a skew deviation (causing vertical diplopia), disconjugate pursuits or nystagmus.7 Although our patient presented to the emergency department with full ocular motilities, remember her son-in-law’s description of the eyes not tracking a target together. It is impossible to know what her motilities looked like at the time of the episode, but it was a very intriguing description.
Finally, strokes involving the visual cortex within the occipital lobe are well known to cause contralateral visual phenomena or vision loss. One study of patients with acute stroke revealed self-limiting visual hallucinations may be relatively frequent in those with occipital lesions. Recall that our patient experienced temporary visual disturbances on the left side. If her vision changes were, in fact, caused by the ischemic lesion in the right occipital lobe, we could surmise that they were likely affecting the left hemisphere bilaterally.
Takeaways
Regardless of the exact etiology behind our patient’s fleeting visual system symptoms, one important point remains: it is of the utmost importance to truly listen to our patients and do our best to understand what they are telling us. It is easy to “write off” symptoms at times, especially when they are transient or not visible to us. However, we must remain vigilant to spot warning signs of disease so we can continue to positively impact the health and well-being of our patients.
Dr. Bozung currently practices at Bascom Palmer where she primarily sees patients in the hospital's 24/7 ophthalmic emergency department. She also serves as the optometry residency program coordinator. Dr. Bozung is a fellow of the American Academy of Optometry and a member of the Florida and American Optometric Associations. She is a founding board member of Young OD Connect and serves on the editorial board for Review of Optometry. She has no financial interests to disclose.
|
1. Constantinescu SM, Maiter D. Pituitary incidentaloma. Presse Med. 2021;50(4):104081. 2. Lee JP, Park IW, Chung YS. The volume of tumor mass and visual field defect in patients with pituitary macroadenoma. Korean J Ophthalmol. 2011;25(1):37-41. 3. Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317(5):516-24. 4. Tamhankar MA, Biousse V, Ying GS, et al. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. 2013;120(11):2264-9. 5. Yokoyama K, Ikeda N, Sugie A, et al. A case of nonapoplectic pituitary adenoma presenting with isolated oculomotor nerve palsy. Asian J Neurosurg. 2021;16(2):391-3. 6. Datar S, Rabinstein AA. Cerebellar infarction. Neurol Clin. 2014;32(4):979-91. 7. Patel VR, Zee DS. The cerebellum in eye movement control: nystagmus, coordinate frames and disconjugacy. Eye (Lond). 2015;29(2):191-5. 8. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12(1):67-76. 9. Wouters A, Dupont P, Christensen S, et al. Association between time from stroke onset and fluid-attenuated inversion recovery lesion intensity is modified by status of collateral circulation. Stroke. 2016;47(4):1018-22. |

