 A 59-year-old man presented for a comprehensive eye examination with a chief complaint of blurry vision in both eyes. His medical history was significant for hypertension and type 2 diabetes, which were both medically controlled. His blood pressure measured 140/84mm Hg, and his last HbA1c level was 7.5%.
A 59-year-old man presented for a comprehensive eye examination with a chief complaint of blurry vision in both eyes. His medical history was significant for hypertension and type 2 diabetes, which were both medically controlled. His blood pressure measured 140/84mm Hg, and his last HbA1c level was 7.5%.
A review of his eye care records revealed a history of bilateral optic nerve head pallor. At visits approximately one and two years earlier, we noted that the patient had pale optic discs in both eyes, as well as constricted visual fields and mildly reduced visual acuity OU. Although we recommended further evaluation on both occasions, the patient did not follow through with testing.
 |
|
 |
|
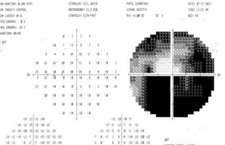
| 1. Central 30-2 visual fields results of our
59-year-old patient. Both the inferior and nasal aspects are severely depressed OU (OD top, OS bottom). |
|
|
|
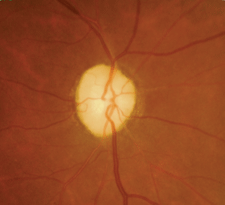
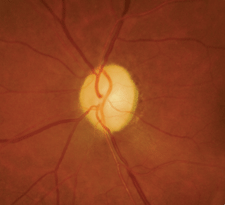
| 2. Dilated funduscopy revealed bilateral optic
nerve head pallor (OD left, OS right). What further testing should we consider? |
Biomicroscopy was unremarkable OU. His intraocular pressure measured 19mm Hg OU. Dilated fundus examination revealed pale optic discs in both eyes, with shallow cupping and slight arteriole attenuation that was consistent with prior diagnostic imagery (figure 2).
Although the patient appeared to have a severe ocular pathology, he was an otherwise robust and active adult who worked fulltime as an athletic coach. How would you proceed in this case? What testing is indicated, and how do you convince this otherwise asymptomatic patient of the need for further evaluation and eventual treatment?
Possible Causes of Pallor
There are numerous potential etiologies of optic nerve pallor, including infarction, infection, infiltration, inflammation, trauma, toxicity, metabolic dysfunction, or direct compression of the nerve or chiasm by a mass lesion.1Ophthalmoscopically, optic atrophy may assume one of several clinical presentations:2
• Primary optic atrophy presents with uniform nerve fiber degeneration, resulting in glial replacement but no architectural alteration of the optic nerve head. The disc appears chalky-white, but the margins remain distinct and the retinal vessels appear normal. Trauma and compression (i.e., secondary to a tumor) typically result in primary optic atrophy.
• Secondary optic atrophy results from pathological disc edema, and may be seen in association with malignant hypertension, papilledema from conditions such as pseudotumor cerebri, or infiltrative diseases like leukemia or sarcoidosis. Further, there is marked degeneration of the neurons with excessive proliferation of glial tissue, indistinct margins and a loss of normal architecture. Typically, the disc appears dirty-gray with ragged edges.
• Consecutive optic atrophy may be seen in degenerative retinal conditions––most notably retinitis pigmentosa, pathological myopia and central retinal artery occlusion. The classic presentation is a pale/waxy disc, normal margins and marked attenuation of the arterioles.
• Glaucomatous optic atrophy presents with a phenomenon known as “cupping,” a progressive excavation of the neuroretinal rim that causes alterations of the disc vasculature, including nasalization and “bayoneting.” Unlike other forms of optic atrophy, however, the rim tissue remains pink and perfused in this condition.
• Temporal disc pallor is the final disorder that must considered in the discussion of optic atrophy. In conditions such as nutritional or demyelinating optic neuropathy, the disc margins remain distinct with normal vasculature; however, the temporal aspect of the nerve acquires a distinctly pale coloration in contrast to the remaining rim tissue. Based upon his clinical appearance, our patient was best described as having primary optic atrophy. But when accounting for his medical history, we considered an ischemic etiology (i.e., anterior ischemic optic neuropathy secondary to hypertension and/or diabetes) in the differential.3
Appropriate Testing
In cases such as this, the most crucial ancillary test is neuroimaging.4 In many instances, visual field defects can help guide the direction of the scan.5 For example, a bitemporal field loss is indicative of chiasmal lesions—while a highly congruous, right homonymous hemianopia typically localizes to the left, post-chiasmal visual pathways in the occipital lobe.In our patient, however, the field loss was so extensive and atypical that it was difficult to predict where the lesion might be. It could be intraorbital, chiasmal or post-chiasmal. Moreover, the clinical presentation might actually be representative of multiple lesions. In these situations, magnetic resonance imaging (MRI) studies of the orbits, the optic chiasm and the brain should be obtained. Contrast dye (gadolinium) is beneficial in discerning malignant lesions.6 MRI also can help identify demyelinating plaques within the white matter of the brain, which are indicative of multiple sclerosis. If MRI fails to reveal any intra-orbital or intracranial abnormalities, then systemic disorders must be investigated.
Numerous conditions can present with visual field loss and optic nerve head pallor. A short list of these systemic disorders includes: sarcoidosis, tuberculosis (TB), Behçet’s disease, lymphoma, leukemia, systemic lupus erythematosus, nutritional or metabolic disorder (e.g., pernicious anemia, folate deficiency), syphilis, Lyme disease and antiphospholipid antibody syndrome. Diabetes and hypertension are other obvious considerations for such a presentation. Hematology, serology and blood chemistry should include a complete blood count (CBC) with white cell differential, erythrocyte sedimentation rate (ESR), angiotensin-converting enzyme (ACE), antinuclear antibody (ANA), serum cardiolipin, serum homocysteine, serum B12 and folate levels, and rapid plasma reagin (RPR) for syphilis.
Additionally, chest X-rays could prove helpful in suspected cases of TB or sarcoidosis. A Mantoux test is confirmatory for TB.
After discussing the findings with our patient and the need for a medical workup, he agreed to see his primary care provider (PCP) for the recommended battery of tests. We sent a summary letter to the PCP on the patient’s behalf and scheduled an appointment for him, but he failed to keep it. We left messages for him explaining the potential urgency of his situation. But, to date, he has neither called nor returned to our office for any medical treatment. It is an unfortunate truth in today’s society, but some individuals simply refuse to accept the help that we offer, even when the potential for morbidity is high. There are many reasons for this—finances, fear and denial are but a few. While we remain concerned and diligent, we tend to operate by a simple code that we have espoused for years: Unless your patient is too young or cognitively impaired to know better, you can’t care more for him than he cares for himself.
1. Sowka JS. Shedding light on a pale optic nerve. Rev Optom. 2011 Nov;148(11):28-36.2. Singh D, Saxena R, Sharma P, Menon V. Systematic approach to a case of disc pallor. Preffered Practice Pattern DJO. 2011;21(3):28-32.3. Nonarteritic ischemic optic neuropathy. In: Lee AG, Brazis PW. Clinical Pathways in Neuro-Ophthalmology: An Evidence-Based Approach, 2nd Ed. New York: Thieme 2003; 73-92.4. Lee AG, Chau FY, Golnik KC, et al. The diagnostic yield of the evaluation for isolated unexplained optic atrophy. Ophthalmology. 2005 May;112(5):757-9.5. Kedar S, Ghate D, Corbett JJ. Visual fields in neuro-ophthalmology. Indian J Ophthalmol. 2011 Mar-Apr; 59(2):103-9.6. Fanea L, Fagan AJ. Review: Magnetic resonance imaging techniques in ophthalmology. Mol Vis. 2012;18:2538-60.7. Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J. 2012 Jan-Apr; 3(1):2-6.

