 |
|
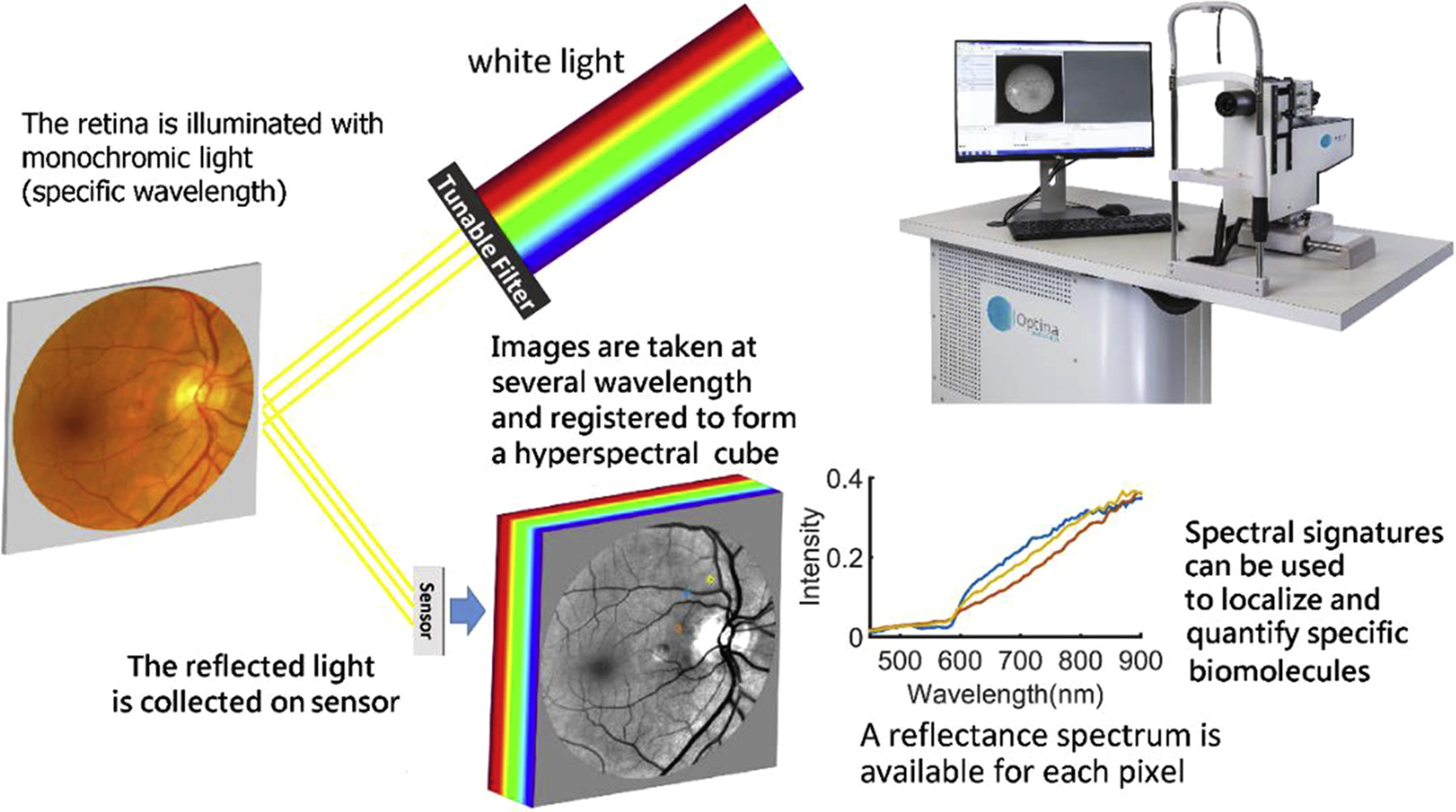
Hyperspectral imaging, though still impractical in a clinical setting, can detect the wavelength “signature” of amyloid beta in the retinas of Alzheimer’s patients. Photo: Alzheimer's Association. Click image to enlarge. |
It’s been known for some time that certain protein deposits implicated in Alzheimer’s disease (AD) have been found to reside in the eye, specifically amyloid beta (Aß) and phosphorylated tau (pTau). A new literature review published in Journal of Neuro-ophthalmology sought to further elucidate the role of the retina as a potential biomarker of the disease.
Gathering literature from PubMed and Google Scholar, the researchers found that curcumin fluorescein and hyperspectral imaging proved as promising methods to detect retinal Aß. While tau aggregation in the retina has been previously limited to histopathologic studies, near-infrared fluorescence imaging, novel Aß labeling techniques and small molecule retinal tau tracers are imaging options that may expand this lack of further evidence.
All these options have their downsides, though, with curcumin-labeled Aß presenting difficulty when trying to distinguish it from background, retinal fluorescence and needing a standardization of dosing and quantification methods. Hyperspectral (HS) imaging is limited by receiving other retinal features’ signals and an observed reflectance variability that exists between individuals.
Reviewing the findings of studies that looked at Aß retinal deposition with curcumin, some presentations were consistent across all. This included the localization of retinal Aß, which appeared in the peripheral, superior temporal quadrant and was consistent with prior histopathological findings. Of note, all studies excluded glaucoma and AMD patients due to potential overlapping fluorescence patterns. Additionally, only one study in this group saw retinal Aß deposition correlated with cognition.
As for HS imaging, it cannot visualize Aß directly, measuring alterations in the reflected light spectra, thought to be caused by small, soluble Aß oligomers possessing Rayleigh light-scattering properties. Because of its indirect nature of measurement, this type of imaging cannot provide Aß information in the forms of quantification or location. Rather, hyperspectral imaging may be better suited to serve as a biomarker of Alzheimer’s in tandem with other features of retinal imaging. This is also in part due to the technique receiving confounding signals from other retinal features. As a result, HS imaging needs a standardized approach, including predetermined ROIs and a way to correct for variability amongst individuals.
Using tau presence in the retina as a potential imaging option is less straightforward. Causing this is the uncertainty of whether retinal tau is the same as tau pathology of the brain. More uncertainty lies in if retinal tau is even associated with clinical and neuropathological displays of Alzheimer’s at all.
Despite this setback, there are new retinal imaging technologies that could still provide application in Aß identification and tau pathology of the retina. Some of these include near-infrared fluorescence imaging, use of nanobodies and fluorescent probes in mice studies. Small molecule retinal tau tracers are an option in early development, but is limited by its required oral or parenteral administration of probes before retinal imaging, compromising their usefulness as a screening device.
Still, the use of tau as a biomarker isn’t off the table, as artificial intelligence has been used as an approach to noninvasively identify its pathology. This, and machine learning models that include data from multiple imaging modalities to predict disease progression, are more likely options when analyzing tau.
As the authors of the study affirm, “Going forward, increasing advanced artificial intelligence-based retinal imaging analysis may be critical to enable early AD diagnosis. HS imaging, fluorescence imaging and novel future imaging techniques will likely be useful inputs in combination with other imaging biomarkers as inputs for these AI-based approaches.”
Tang MY, Blazes MS, Lee CS. Imaging amyloid and tau in the retina: Current research and future directions. J Neuro-Ophthalmol. 2023. [Epub ahead of print]. |

