Age-related macular degeneration (AMD) is one of the leading causes of blindness in older individuals. Blindness secondary to AMD is irreversible and can have a catastrophic impact upon a patient’s daily activities. Additionally, AMD poses a significant burden on worldwide health care costs. Therefore, when managing patients with AMD, prevention of progressive damage and maintenance of visual function remain the fundamental treatment goals.
To this accord, we have seen a recent explosion in research studies that are intended to identify primary risk factors, as well as solidify an understanding of the pathogenesis and progression of AMD. Here, we will review and compare both the original Age-Related Eye Disease Study (AREDS) and AREDS2, and examine how results from both studies have impacted and will impact the management of patients with AMD. In conjunction, we will examine the existing body of literature on the specific role of carotenoids and omega-3 long chain polyunsaturated fatty acids in the prevention of AMD.
AREDS and AREDS2
AREDS, a landmark research project that evaluated the natural history of AMD and cataract, featured a controlled, randomized clinical trial that evaluated the effect of pharmacological doses of nutritional supplements on the rate of progression to advanced AMD.1 The researchers evaluated the protective effects of zinc and/or a formulation that consisted of nutrients with antioxidant properties, including vitamin C, vitamin E and beta-carotene. The researchers concluded that a combination of these antioxidants with zinc led to an overall 25% risk reduction in disease progression in individuals who had a moderate risk of AMD development.1 The overall risk of moderate vision loss (defined as greater than or equal to 15 letters by the Early Treatment of Diabetic Retinopathy Study) was reduced by 19% at five years.1
However, like many other groundbreaking studies, the original AREDS researchers generated numerous unanswered questions and suggestions for areas of further study. Many of these items have already been or will be addressed by AREDS2. The enrollment for AREDS2 concluded in June 2008, and participants will be followed for five to six years. The first official reports from AREDS2 are anticipated in 2013.2
The primary objective of AREDS2 is to evaluate the effect of dietary carotenoids—including lutein and zeaxanthin—and/or omega-3 fatty acids—including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)—on the progression to advanced AMD.3 In an ideal world of healthy living, these dietary compounds would be best obtained through a balanced diet. In reality, however, our food intake is severely deficient of these important nutrients.
The average western diets contain 1.3mg to 3mg of lutein and zeaxanthin, with a ratio of lutein to zeaxanthin of 7:1.4,5 The highest natural concentrations of lutein are found in dark green leafy vegetables, such as spinach, kale and collard greens, whereas zeaxanthin is the major carotenoid found in corn, orange peppers and oranges. Additionally, both lutein and zeaxanthin are found in a high-mole percentage in egg yolk.6 (One additional carotenoid, meso-zeaxanthin, is found in shrimp, fish skin and turtle fat.7) It is important to note that approximately 78% of dietary lutein and zeaxanthin is sourced from vegetables.6
Of the numerous carotenoids found in nature, just lutein, zeaxanthin and meso-zeaxanthin are present in human macular pigment.8 Because these carotenoids are likely to play a part in the maintenance of normal cell physiology, the absence or reduced bioavailability of either one may account for the development of the structural changes that occur in patients with AMD. Both lutein and zeaxanthin were considered for study in AREDS; however, neither carotenoid was commercially available for manufacturing in a research formulation.3 However, AREDS2 researchers are studying their efficacy in preventing the progression of macular degeneration in subjects who present with intermediate drusen.
Lutein, zeaxanthin and meso-zeaxanthin represent the majority of all retinal carotenoids. Both lutein and zeaxanthin are garnered from dietary intake; however, there is strong evidence to suggest that meso-zeaxanthin primarily is formed during chemical reactions within the retina.9 Richard Bone, Ph.D., and associates also noted that, although the total plasma levels of lutein exceed the levels of zeaxanthin in the human body, there actually is more zeaxanthin/meso-zeaxanthin in the retina than lutein (particularly in the macula).8,9
AREDS2 and AMD
AMD is a major cause of blindness among individuals with European ancestry and accounts for more than 50% of all blinding conditions.10 The Eye Disease Prevalence Research Group estimates that 1.2 million U.S. residents are living with neovascular AMD and 970,000 have geographic atrophy; these numbers expected to rise 50% by the year 2024.10
There are three major purported risk factors for AMD: age, positive family history and smoking. In fact, smoking is the only consistently proven risk factor for AMD.11-14 As with other age-related diseases, the free radical theory of aging has been proposed as an underlying cause of AMD. This theory proposes that oxidative stress occurs in the retina because levels of reactive oxidative intermediates (ROIs) increase to a level that exceeds the detoxifying capacity of antioxidants.
Animal experiments have shown that zeaxanthin can markedly alter and inhibit oxidative damage in diabetic rats, whereas lutein treatment led to clinically significant suppression of choroidal neovacularization development and inflammation.15-18 In other words, the macular carotenoids are capable of quenching the ROIs, thus protecting the retinal pigment epithelium (RPE). In addition to the quenching of ROIs, the carotenoids that form macular pigment optical density (MPOD) may protect the eye by absorption of blue light, which has been shown to catalyze drusen formation and AMD development. (The macular pigment has both vertical and horizontal orientation within the photoreceptor and the outer plexiform layer, which maximizes the prereceptoral absorption of incident blue light.19,20)
We have now learned that diets rich in lutein and zeaxanthin have been shown to protect against the development of AMD.21-24 Oral supplementation of lutein or lutein/zeaxanthin combinations in individuals at risk for AMD development and control subjects increased mean serum levels of the carotenoid metabolites that constitute the macular pigment. These effects are not always uniform in all individuals.25-29 Unfortunately, however, AREDS2 researchers will not be evaluating MPOD levels in study participants.25-29
Nutrient Testing in AREDS2
• Omega-3 long chain polyunsaturated fatty acids. One important question being addressed by AREDS2 is whether high supplemental doses of omega-3 fatty acids will inhibit the development of advanced AMD. Researchers have previously determined that DHA and EPA may serve as cytoprotective agents against several retinal diseases as well as cataracts.30 DHA is a major component of retinal photoreceptor outer segment membranes, and an insufficiency of this essential fatty acid can result in altered retinal function.31-35
Overall, epidemiological studies have shown a higher prevalence of AMD in individuals with diets high in total fat intake (exempting diets that are specifically high in omega-3 fatty acids).36-43 Using the Harvard Dietary Assessment, AREDS researchers found that a dietary intake of omega-3 fatty acids was associated with a reduced likelihood of progression to central geographic atrophy in individuals with bilateral drusen at baseline.44 AREDS2 researchers will further investigate this association by experimentally controlling the variables and providing consistently defined dosages.
• Zinc. Another important supplement investigated in AREDS was zinc, which, dosed at 80mg per day, was shown to be associated with decreased risk for mortality.45 However, one subsequent study indicated that patients with higher levels of zinc in their aqueous humor were more likely to develop cataracts than healthy controls.46 Additionally, there was a significant increase in the number of hospitalizations for genitourinary causes in AREDS participants who received zinc supplementation at high doses of 80mg per day.47
The tolerable upper level of daily zinc intake is 40mg; however, AREDS researchers supplemented patients with 80mg of zinc per day.44,45 AREDS2 investigators are examining the effects of both 80mg and 40mg dosages of zinc per day to determine whether a lower dose could still prevent the development or progression of AMD.
• Beta-carotene. AREDS2 will also investigate the effect of eliminating beta-carotene from the original AREDS formulation. This is of particular interest because high doses of beta carotene alone, or in combination with vitamin E, are associated with a statistically significant risk of developing lung cancer in smokers.48,49 Therefore, it is not recommended to place any patients who smoke or recently quit smoking on beta carotene supplementation.
AREDS2, To Date
AREDS2 researchers have enrolled more than 4,000 participants, and the study is intended to detect at least 25% difference between the placebo group and the respective treatment groups regarding progression to advanced AMD.3 AREDS2 participants were assigned to randomly take one of the following daily supplement formulations:
• Placebo for both lutein/zeaxanthin and EPA/DHA.
• 10mg lutein/2mg zeaxanthin and EPA/DHA placebo.
• Placebo for lutein/zeaxanthin and 350mg DHA/650mg EPA.
• 10mg lutein/2mg zeaxanthin and 350mg DHA/650mg EPA.
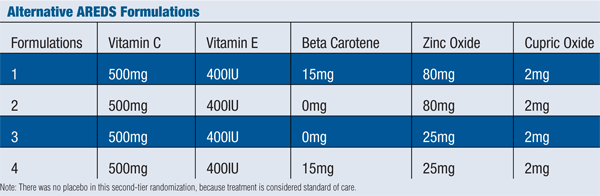
Further, all participants were offered the original AREDS formulation, because it is still considered standard of care. Those who agreed to take the AREDS formulation and consented to a second randomization were given one of four alternative AREDS formulations in addition to the study supplements described above (see “Alternative AREDS Formulations,” above).3
A reduction in the amount of zinc oxide and complete elimination of the beta carotene served as variables in the alternative formulations.
All potential participants in AREDS2 were questioned regarding their smoking habits. For participants who were smokers or had quit smoking within the past year, randomization into the beta-carotene formulations was not permitted. (This was the case regardless whether the participant agreed to the second level of randomization.)
Final Considerations
As always, the unique nature of individualized dietary and lifestyle habits can pose significant difficulty when garnering accurate measurements of nutrient consumption. Healthy dietary choices make a lifelong impact, and the benefits of proper nutrient intake may begin as early as fetal development. For example, pregnant mothers who consume lower amounts of fruits and vegetables have a higher risk of a child with sporadic retinoblastoma.50 Additionally, extensive sunlight exposure throughout an individual’s lifetime is associated with AMD development.51,52 As the eye ages, yellowing of the crystalline lens acts as a natural filter to blue light; however, this means that the majority of retinal exposure to sunlight occurs at an early age when individuals are less likely to be on prophylactic therapies. So, it is important for everyone to establish healthy eating habits and begin using nutritional supplements as early as possible.
The million-dollar question: Have we now officially determined that dietary and lifestyle changes at an early age can reduce the risk for AMD development and progression? The answer is quite clear: Yes. Results from AREDS and other nutritional studies strongly indicated that the earlier an individual begins to make lifestyle alterations and take nutritional supplements, the less risk he or she has for degenerative eye disease. And, for older patients who may already be at an increased risk for AMD, it is better late than never when it comes to the use of nutraceuticals. In the very near future, the results from AREDS2 will shed more light on the most effective management options for AMD as well as address which specific nutritional supplements are most useful in disease prevention and treatment.
Dr. Gunvant is an assistant professor at Southern College of Optometry in Memphis, Tenn., and an adjunct assistant professor at the University of Louisville and the University of Memphis.
Dr. Jennings is the site coordinator for AREDS2 at the University of Tennessee Health Science Center. She also works on various research studies in the Retinal Degenerations and Ophthalmic Genetics Service.
Mrs. Vyas is a registered dietitian, licensed dietitian and nutritionist. She currently practices at the Provena St. Joseph Hospital in Elgin, Ill.
Thanks to Dr. Dennis Gerhart, chairman and chief scientific officer for ZeaVision, LLC, for clinical input and Miss Anna Ablamowicz for her assistance with the literature search.
1. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36.
2. Age-Related Eye Disease Study 2. Welcome to AREDS2. Available at:
www.areds2.org/ (Accessed February 12, 2011).
3. Chew E. Age-related eye disease study 2 protocol. Available at: https://web.emmes.com/study/areds2/resources/areds2_protocol.pdf (Accessed February 12, 2011).
4. Nebeling LC, Forman MR, Graubard BI, et al. Changes in carotenoid intake in the United States: The 1987 and 1992 National Health Interview Surveys. J Am Diet Assoc. 1997 Sep;97(9):991-6.
5. Nebeling LC, Forman MR, Graubard BI, et al. The impact of lifestyle characteristics on carotenoid intake in the United States: The 1987 National Health Interview Survey. Am J Public Health. 1997 Feb;87(2):268-71.
6. Sommerburg O, Keunen JE, Bird AC, et al. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998 Aug;82(8):907-10.
7. Maoka T, Arai A, Shimizu M, Matsuno T. The first isolation of enantiomeric and mesozeaxanthin in nature. Comp Biochem Physiol B. 1986;83(1):121-4.
8. Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25(11):1531-5.
9. Bone RA, Landrum JT, Hime GW, et al. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993 May;34(6):2033-40.
10. Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004 Apr;122(4):564-72.
11. Loane E, Kelliher C, Beatty S, Nolan JM. The rationale and evidence base for a protective role of macular pigment in age-related maculopathy. Br J Ophthalmol. 2008 Sep;92(9):1163-8.
12. Klein R, Klein BEK, Moss SE. Relation of smoking to the incidence of age-related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol. 1998 Jan;147(2):103-10.
13. Tan JS, Mitchell P, Kifley A, et al. Smoking and the long-term incidence of age related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2007 Aug;125(8):1089-95.
14. Chakravarthy U, Augood C, Bentham GC, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology. 2007 Jun;114(6):1157-63.
15. Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1645-51.
16. Izumi-Nagai K, Nagai N, Ohgami, et al. Macular pigment leutin is anti-inflammatory in preventing choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2555-62.
17. Kirschfeld K. Carotenoid pigments: their possible role in protecting against photooxidation in eyes and photoreceptor cells. Proc R Soc Lond B Biol Sci. 1982 Aug;216(1202):71-85.
18. Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997 Aug;38(9):1802-11.
19. Sujak A, Gabrielska J, Grudzinski W, et al. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch Biochem Biophys. 1999 Nov;371(2):301-7.
20. Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006 Sep-Oct;51(5):461-81.
21. Gale CR, Hall NF, Phillips DI, Martyn CN. Leutein and zeaxanthin statusand risk of age related macular degeneration. Invest Ophthalmol Vis Sci. 2003 Jun;44(6):2461-5.
22. Delcourt C, Carriere I, Delage M, et al. Plasma leutein and zeaxanthin and other carotenoids as modifiable reisk factors for age-related maculopathy and cataract. Invest Ophthalmol Vis Sci. 2006 Jun;47(6):2329-35.
23. Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age-related macular degeneration and leutin and zeaxanthin in the carotenoids in age-related eye disease study (CAREDS). Arch Ophthalmol. 2006 Aug;124(8):1151-62.
24. Tan JS, Wang JJ, Flood V, et al. Dietary antioxidants and long-term incidence of age-related macular degeneration. The Blue Mountains Eye Study. Ophthalmology. 2008 Feb;115(2):334-41.
25. Berendschot TT, Goldbohm RA, Klöpping WA, et al. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci. 2000 Oct;41(11):3322-6.
26. Khachik F, de Moura FF, Chew EY, et al. The effect of lutein and zeaxanthin supplementation on metabolites of these carotenoids in the serum of persons aged 60 or older. Invest Ophthalmol Vis Sci. 2006 Dec;47(12):5234-42..
27. Koh HH, Murray IJ, Nolan D, et al. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Exp Eye Res. 2004 Jul;79(1):21-7.
28. Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res. 2007;84:718-28.
29. Richer S, Devenport J, Lang JC. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry. 2007 May;78(5):213-9.
30. SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005 Jan;24(1):87-138.
31. Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22(2):79-131.
32. Neuringer M. In: Lipids, Learning, and the Brain: Fats in Infant Formulas. 103rd Ross Conference on Pediatric Research. Dobbing J (ed.). Adeliade, South Australia: Ross Laboratories; 1993:134-58 37.
33. Schaefer EJ, Robins SJ, Patton GM, et al. Red blood cell membrane phosphatidylethanolamine fatty acid content in various forms of retinitis pigmentosa. J Lipid Res. 1995 Jul;36(7):1427-33.
34. Hoffman DR, Birch DG. Docosahexaenoic acid in red blood cells of patients with X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1995 May;36(6):1009-18.
35. Hoffman DR, Uauy R, Birch DG. Metabolism of omega-3 fatty acids in patients with autosomal dominant retinitis pigmentosa. Exp Eye Res. 1995 Mar;60(3):279-89.
36. Mares-Perlman JA, Brady WE, Klein R, et al. Dietary fat and age-related maculopathy. Arch Ophthalmol. 1995 Jun;113(6):743-8.
37. Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish. Arch Ophthalmol. 2003 Dec;121(12):1728-37.
38. Cho E, Hung S, Willett WC, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr. 2001 Feb;73(2):209-18.
39. Smith W, Mitchell P, Leeder SR. Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol. 2000 Mar;118(3):401-4.
40. Heuberger RA, Mares-Perlman JA, Klein R, et al. Relationship of dietary fat to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Arch Ophthalmol. 2001 Dec;119(12):1833-8.
41. Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age related macular degeneration. Arch Ophthalmol. 2001 Aug;119(8):1191-9.
42. Chua B, Flood V, Rochtchina E, et al. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch Ophthalmol. 2006 Jul;124(7):981-6.
43. Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006 Jul;124(7):995-1001.
44. SanGiovanni JP, Chew EY, Agron E, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report No. 23. Arch Ophthalmol. 2008 Sep;126(9):1274-9.
45. Clemons TE, Kurinij N, Sperduto RD, et al. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13. Arch Ophthalmol. 2004 May;122(5):716-26.
46. Nourmohammadi I, Modarress M, Pakdel F. Assessment of aqueous humor zinc status in human age-related cataract. Ann Nutr Metab. 2006;50(1):51-3.
47. Johnson AR, Munoz A, Gottlieb JL, Jarrard DR. High dose zinc increases hospital admissions due to genitourinary complications. J Urol. 2007 Feb;177(2):639-43.
48. Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996 Nov;88(21):1550-9.
49. Albanes D, Heinonen OP, Huttunen JK, et al. Effects of alphatocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995 Dec;62(6 Suppl):1427S-1430S.
50. Orjuela MA, Titievsky L, Liu X, et al. Fruit and vegetable intake during pregnancy and risk for development of sporadic retinoblastoma. Cancer Epidemiol Biomarkers Prev. 2005 Jun;14(6):1433-40.
51. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age related macular degeneration. Mol Vis. 1999 Nov;5:32.
52. Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006 Feb;84(1):4-15.

